Abstract
Background
The rate of adequate cleansing (ACR) and complete examinations (CR) are key quality indicators in capsule colonoscopy (CC) and pan-intestinal capsule endoscopy (PCE).
Aims
To evaluate the efficacy of bowel preparation protocols regarding ACR and CR.
Methods
We conducted a systematic review and meta-analysis, search terms regarding colon capsule preparation, publication date from 2006/01, and date of search 2021/12, in six bibliographic databases. Multiple steps of the cleansing protocol were assessed: diet, adjunctive laxatives, purgative solution, use of prokinetic agents, and “booster”. The meta-analytical frequency of ACR and CR was estimated, and subgroup analyses performed. Strategies associated with higher ACR and CR were explored using meta-analytical univariable and multivariable regression models.
Results
Twenty-six observational studies and five RCTs included (n = 4072 patients). The pooled rate of ACR was 72.5% (95% C.I. 67.8–77.5%; I2 = 92.4%), and the pooled rate of CR was 83.0% (95% C.I. 78.7–87.7%; I2 = 96.5%). The highest ACR were obtained using a low-fibre diet [78.5% (95% C.I. 72.0–85.6%); I2 = 57.0%], adjunctive laxatives [74.7% (95% C.I. 69.8–80.1%); I2 = 85.3%], and split dose < 4L polyethylene glycol (PEG) as purgative [77.5% (95% C.I. 68.4–87.8%); I2 = 47.3%]. The highest CR were observed using routine prokinetics prior to capsule ingestion [84.4% (95% C.I. 79.9–89.2%); I2 = 89.8%], and sodium phosphate (NaP) as “booster” [86.2% (95% C.I. 82.3–90.2%); I2 = 86.8%]. In univariable models, adjunctive laxatives were associated with higher ACR [OR 1.81 (95% C.I. 1.13; 2.90); p = 0.014]. CR was higher with routine prokinetics [OR 1.86 (95% C.I. 1.13; 3.05); p = 0.015] and split-dose PEG purgative [OR 2.03 (95% C.I. 1.01; 4.09), p = 0.048].
Conclusions
Main quality outcomes (ACR, CR) remain suboptimal for CC and PCE. Despite considerable heterogeneity, our results support low-fibre diet, use of adjunctive sennosides, split dose < 4L PEG, and routine prokinetics, while NaP remains the most consistent option as booster.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Capsule endoscopy currently allows for non-invasive endoscopic evaluation of both the small bowel and colon. It has been a field of active research, with the development of the double-camera PillCam COLON® (first and second generation released in 2006 and 2009, respectively) and the PillCam Crohn’s® capsule (2017), as swallowable wireless miniature cameras, with approximately the size of a pill (31.5 × 11.6 mm) that enable non-invasive colonoscopy plus the acquisition of endoscopic images of the entire small bowel, setting a privileged field (as “small bowel-colon capsules”) for a new concept of “pan-intestinal capsule endoscopy” (PCE). These capsules have two cameras in opposite sides, with a wide field of view (2 × 172°), a high frame rate of image capture (up to 35 endoscopic images per second) and extended battery duration (more than 12 h), and were demonstrated to be safe and effective, ensuring high diagnostic accuracy both for the small bowel and the colon mucosal evaluation [1,2,3]. To date, they have mostly been used in cases of previous incomplete conventional colonoscopy (due to redundant colon, loops, or acute bends preventing successful cecum intubation) or in patients with inflammatory bowel disease, mainly Crohn’s Disease involving both the small bowel and the colon [4,5,6].
While the small bowel is usually effectively clean with standard bowel preparation protocols [2], adequate colonic cleansing remains a challenge. An optimized bowel preparation is pivotal to ensure an effective colon capsule (CC) examination, as it is not possible to wash or aspirate debris during the examination. In fact, a suboptimal bowel preparation may render the examination inconclusive, with a high burden both to the patient and the healthcare system. However, there is currently no consensus on the optimal bowel preparation protocol for CC or PCE to ensure effective colon cleansing (ACR) and completeness rates (CR). Variables such as the pre-procedure diet, type, and volume of the purgative solution, type, and volume of the “booster”, as well as the use of adjunctive laxatives (such as sennosides) or prokinetic drugs (such as metoclopramide or domperidone) prior to capsule ingestion, are likely to play an important role. However, the evidence in the literature addressing this issue has only been scarcely assessed.
The aim of this systematic review and meta-analysis is to synthesize and analyse the available evidence towards the optimal bowel preparation protocol for CC or PCE, regarding adequate cleansing rate (ACR) and complete examinations rate (CR).
Methods
We performed a systematic review of which methodology followed the guidance of the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) [7]. The review protocol was registered with the International Prospective Register of Systematic Reviews by the Centre for Reviews and Dissemination at the University of York (PROSPERO), with the ID: CRD42020176645.
Inclusion Criteria
Primary studies to be included in this systematic review were those investigating adult patients (≥ 18 years old) submitted to capsule colonoscopy or PCE with a double-headed capsule (PillCam COLON 1®, PillCam COLON 2® or PillCam Crohn’s®), independently of the bowel preparation protocol and the clinical indication for the exam. Eligible studies included prospective or retrospective cohort studies, randomized controlled trials, case–control studies, or case series. We excluded smaller studies, with less than 20 patients. Case reports, reviews, opinion articles, and systematic reviews or meta-analyses were also excluded.
Studies were only included if available information indicated the bowel preparation regimen used, and if data on the efficacy outcomes (ACR; CR) were clearly reported. CR corresponded to capsule being expelled or reaching the hemorrhoidal pedicles within the battery time. For the definition of “adequate bowel preparation”, we considered the definition used by the authors of original articles, independently of the cleansing scale or qualitative classification used for evaluation and report. The Leighton-Rex capsule colonoscopy cleansing scale was used by default, as a 2-point scale (adequate versus inadequate) or a 4-point scale (poor, fair, good and excellent) where applicable [8], decreasing data heterogeneity.
Information Sources and Search Strategy
Searches were conducted from 2006, the year of release of the CC in the market and start of use in clinical practice, until December 2021, in six electronic databases: PubMed, CINAHL (via EBSCO), EMBASE (via Elsevier), and CENTRAL: Cochrane Central Register of Controlled Trials (via Wiley Online Library) and Web of Science Core Collection (via Clarivate Analytics), clinicaltrials.gov, OpenGrey, and Grey Literature Network Service. In addition, hand searches of the reference lists of all included studies and previously published systematic reviews of bowel preparation for CC or PCE were performed. No geographical restriction was applied. The search strategy was developed in consultation with a medical librarian with expertise in systematic review searching. The search terms were adjusted to the specificities of the different databases. The full query is available in Supplementary Table 1.
Selection of Studies
After duplicates’ removal, two reviewers independently screened the titles and abstracts to identify potentially eligible studies, and subsequently performed full-text review of those filtered to determine inclusion. Reviewers were not blinded to the study authors, institutions, or publication sites.
Data Extraction and Coding
Two reviewers independently extracted relevant data from each selected study, using a standardized data extraction electronic form: diet type (low fibre versus clear liquids), use of adjunctive laxatives prior to the purgative solution (none versus sennosides), purgative solution volume (standard PEG 4L versus < 4L), and/or type (standard PEG versus low-volume PEG-ascorbate), prokinetic agents (used routinely before capsule ingestion versus selectively only in patients with delayed gastric passage of the capsule as assessed with real-time viewer), and the “booster” (standard sodium phosphate versus oral sulphate solution (OSS), PEG-based or gastrografin-based). In addition, we extracted data on the sampling method, number of participants, and number of patients with ACR and CR. If the same data had been reported in multiple study publications, the duplicates were deleted, to minimize the overrating of any variable or outcome investigated in the same sample.
Quality Assessment
Potential bias of the included studies was evaluated by two independent investigators, using a scoring system proposed by Rokkas et al. [9, 10]—Supplementary Table 2. We considered studies scoring 4/5 and above as high quality and 3/5 as moderate risk of bias. Studies scoring 2/5 and below were considered as being at high risk of bias and were excluded.
Disagreements between reviewers were resolved through consensus or consultation with a third reviewer if required.
Statistical Analysis
For each primary study, we registered data regarding the protocol variables and outcomes on an individual participant basis. We performed a random-effects meta-analysis of log-transformed proportions to estimate the pooled frequency of patients achieving adequate cleansing or a clean preparation. Meta-analytical log-transformed proportions were back-transformed in the natural scale. Heterogeneity was assessed using the I2 statistic, and the Q-Cochran test p value—an I2 > 50% and a p value < 0.10 were deemed to represent substantial heterogeneity. Subgroup analyses were performed according to the different protocol strategies.
In addition, to identify sources potentially associated with improved cleansing or completeness, we applied a reduced random-effects one-stage model to identify the association between each protocol strategy and the obtention of a complete examination or clean preparation. To assess the robustness of our results, we performed a sensitivity analysis, applying a fully bivariate random-effects one-stage model. Models were described as applied by Debray et al. [11] Exponentials of the regression coefficients were interpreted as odds ratio (OR). In case that, in univariable models, more than one variable achieved marginal association (p < 0.20) with the outcome, multivariable models were built. All analyses were performed using software R.
Results
We identified a total of 79 eligible studies through database searching—Fig. 1: PRISMA flow diagram. Supplementary Table 3 details the rationale for study selection. Thirty-one studies were considered to have good methodological quality, with low or moderate risk of bias, being selected for systematic review and meta-analysis—Table 1. This included five randomized controlled trials (RCTs) [12,13,14,15,16] and 26 observational studies [1, 5, 17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40], with a total of 4072 patients included. Regarding the RCTs, each study group was considered as an individual cohort for the purpose of data analysis, finally resulting in 39 study protocols depicted for quantitative synthesis. Table 1 summarizes identification, recruitment methodology, type of capsule, sample size, bowel preparation protocol, and main outcomes for each of the studies included. Approximately half of the studies (14/31, 45.2%) were multicentric [1, 15, 16, 18, 22, 23, 25, 29, 30, 34,35,36,37, 40].
Descriptive Results
Distribution of Study Population per Bowel Preparation Protocol Variables
Supplementary Figure 1 represents the total number of patients on the different bowel preparation protocols, for each of the five main domains (diet, adjunctive laxative, purgative solution, prokinetic, and booster).
Diet Thirty-one study protocols [1, 5, 12,13,14,15,16,17,18,19,20,21,22,23,24,25,26, 28, 32, 34,35,36,37,38, 40] followed a clear liquid diet (n = 3437 patients), while 8/39 protocols [14, 27, 29,30,31, 33, 39] (n = 635 patients) allowed a low-fibre diet the day before the procedure.
Adjuvants Adjunctive laxatives were used at the first stage of the bowel preparation protocol, prior to ingestion of the purgative solution, in 19/39 protocols [1, 5, 12,13,14,15,16, 22, 26, 28, 29, 31, 33, 36, 39] (n = 2224 patients). Adjunctive laxatives corresponded mostly to four 12 mg sennosides tablets [1, 5, 12, 13, 16, 22, 28, 36], while fewer protocols used bisacodyl [14, 39], sodium picosulphate [29], sodium bicarbonate [31], mosapride [33], or magnesium tablets [15].
Prokinetics Domperidone or metoclopramide was used with the purpose of reducing the gastric transit time of the capsule in all but two [34, 36] protocols. It was used routinely (in all patients prior to capsule ingestion) in 26 protocols [12,13,14,15, 17,18,19,20,21, 23,24,25,26,27,28,29,30,31, 33] (n = 1406 patients), and selectively in 11 protocols [1, 5, 16, 22, 32, 35, 37,38,39,40] (n = 2408 patients). Domperidone (most often given in a single dose of 20 mg) was the prokinetic of choice in 22/39 (56.4%) protocols [5, 12,13,14,15, 17,18,19,20,21,22,23, 26, 28, 29, 38], the administration being selective in 3/22 (13.6%) of cases [5, 22, 38]. Metoclopramide (mostly a 10 mg single dose) was used in 12/39 (30.8%) protocols [1, 16, 25, 30,31,32, 35, 37, 39, 40], the administration being selective in 8/12 (66.7%) of cases [1, 16, 32, 35, 37, 39, 40]. Less frequently, mosapride 5–20 mg [29, 31, 33], tegaserod 6+6 mg [24], itopride 50 mg [27], or erythromycin 250 mg [16] was used as prokinetics.
Simethicone (100–200 mg) was used prior to capsule ingestion in only 3/39 (7.7%) of all study arms [31, 33, 38], with the purpose of reducing the amount of bubbles that may limit mucosal visibility.
Purgative solution In all studies included, the purgative was taken in a split-dose schedule, the first dose being administered the evening before, and the second dose the early morning in the day of the procedure. The purgative solution was polyethylene glycol (PEG) 4L (i.e. 2+2 or 3+1L) in 22 protocols [1, 5, 12,13,14, 16, 17, 19,20,21,22, 25, 26, 28, 32, 35,36,37, 40] (n = 2866 patients), PEG less than 4L (1.5–3L) in 5 [14, 24, 27, 31, 33] (n = 138), and low volume PEG-ascorbate (PEG-Asc) in 12 protocols [13, 15, 23, 29, 30, 34, 38, 39] (n = 1068 patients).
Boosters Additional boosters were used in all protocols, being administered at two stages, the first dose upon detection of the capsule passage to the small bowel, and the second dose usually 2–3 h after the first dose, with the main goal of accelerating the transit time of the capsule while contributing to maintain a clear liquid-filled intestinal lumen. The effect of the boosters also required variable amounts of additional free water, up to 1L after each dose, with the purpose of simultaneously increasing effectiveness and safety. The booster was sodium phosphate (NaP) in 22/39 (56.4%) protocols [12,13,14, 17,18,19,20,21,22, 24,25,26,27,28, 30, 32, 34, 38, 40, 41] (n = 1908), administered in two separate doses of 30–45 mL and 15–25 mL (first and second dose, respectively). Oral sulphate solution (OSS) (up to 250 mL per dose) was the booster used in 5/39 (12.8%) protocols [1, 15, 16, 37, 39] (n = 1478). A PEG-based booster was used in 7/39 (17.9%) protocols [12, 15, 23, 30, 31, 33] (n = 213 patients), either PEG-only [12, 33] or associated with ascorbate (PEG-Asc 500–750 mL followed by second dose 250–330 mL) [15, 23, 30, 34] or magnesium citrate [31]. Gastrografin-based solutions were used as booster in 5/39 (12.8%) protocols [5, 15, 16, 29, 36] (n = 473 patients); gastrografin dose was 50–60 mL and 25–30 mL for first and second doses, respectively, in every case being associated with some supplementary booster: NaP [5, 36], OSS [16], PEG-Asc [15], or magnesium citrate/mosapride [29].
Finally, a 10 mg bisacodyl suppository was administered as a “rescue laxative” 1–2 h after the second booster if the capsule had not been excreted, in all but 5/39 (87.2%) protocols [27, 31, 33, 39]; one study used glycerine suppository for this purpose [35]. A light snack was allowed in 13/39 (33.3%) protocols [1, 12, 15, 17,18,19, 21, 24, 26, 28, 31, 33], generally following the bisacodyl suppository administration.
Meta-analytical Results
Quality of Cleansing
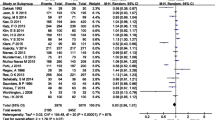
Overall, the meta-analytical proportion of patients achieving adequate (good or excellent) cleansing was of 72.5% (95% C.I. 67.8–77.5%). However, severe heterogeneity was found (I2 = 92.4%). Supplementary Figure 2 represents the forest plot for the overall proportion of adequate cleansing. “Leave-One-Out” sensitivity analysis for adequate cleansing is represented in Supplementary Table 4.
Figure 2 and Table 2 summarize subgroup analyses for ACR per protocol variable.
Diet ACR was 70% (95% C.I. 65.2–75.3%, p < 0.001) in patients drinking only clear liquids [1, 5, 12,13,14,15,16,17,18,19,20,21,22,23,24,25,26, 28, 32, 34,35,36,37,38, 40] (n = 3437) versus 78.5% (95% C.I. 72.0–85.6%, p = 0.019) in those who were allowed to have a low-fibre diet [14, 27, 29,30,31, 33, 39] (n = 635 patients) the day before the procedure.
Adjuvants ACR was 74.7% (95% C.I. 69.8–80.1%, p < 0.001) in patients taking adjunctive laxatives [1, 5, 12,13,14,15,16, 22, 26, 28, 29, 31, 33, 36, 39] (n = 2224 patients), versus 68.4% (95% C.I. 61.8–75.6%, p < 0.001) in those patients where no pre-purgative adjuncts were used [12, 14, 17, 18, 20, 21, 23,24,25, 27, 30, 32, 34, 35, 37, 38, 40] (n = 1848).
Prokinetics ACR was achieved in 70.6% (95% C.I. 65.2–76.3%, p = 0.878) when no prokinetics were used [34, 36] (n = 258), versus 68.8% (95% C.I. 60.0–78.9%, p < 0.001) when a prokinetic was used selectively [1, 5, 16, 22, 32, 35, 37,38,39,40] (n = 2408), and 73.2% (95% C.I. 68.2–78.5%, p < 0.001) when it was used routinely in every patient prior to capsule ingestion [12,13,14,15, 17,18,19,20,21, 23,24,25,26,27,28,29,30,31, 33] (n = 1406).
Purgative solution ACR was obtained in 67.0% (95% C.I. 58.2–77.0%, p < 0.001) of patients receiving the PEG-Asc [13, 15, 23, 29, 30, 34, 38, 39] (n = 1068), versus 77.5% (95% C.I. 68.4–87.8%, p = 0.109) of those having < 4L PEG [14, 24, 27, 31, 33] (n = 138), and 72.9% (95% C.I. 67.8–78.3%, p < 0.001) of those drinking the 4L PEG solution [1, 5, 12,13,14, 16, 17, 19,20,21,22, 25, 26, 28, 32, 35,36,37, 40] (n = 2866).
Boosters ACR was 70.5% (95% C.I. 63.9–77.8%, p < 0.001) for NaP [12,13,14, 17,18,19,20,21,22, 24,25,26,27,28, 30, 32, 34, 38, 40, 41] (n = 1908), 71.5% (95% C.I. 61.5–83.0%, p = 0.002) for PEG-based [12, 15, 23, 30, 31, 33] (n = 213 patients), 71.1% (95% C.I. 66.9–75.6%, p = 0.053) for OSS [1, 15, 16, 37, 39] (n = 1478), and 74.2% (95% C.I. 64.5–85.2%, p < 0.001) for gastrografin-based solutions [5, 15, 16, 29, 36] (n = 473).
Table 3 describes the results of the univariable analyses identifying the association between each protocol option and the occurrence of ACR. The use of an adjunctive laxative prior to ingestion of the purgative solution was significantly associated with a higher rate of adequate cleansing [OR 1.81 (95% C.I. 1.13; 2.90), p = 0.014]. The graphical summary of results is represented in Fig. 4.
Completeness
Overall, the meta-analytical proportion of cases with complete colonic evaluation was of 83.0% (95% C.I. 78.7–87.7%), but with severe heterogeneity being observed (I2 = 96.5%). Supplementary Figure 3 represents the forest plot for the overall proportion of completeness. “Leave-One-Out” sensitivity analysis for completeness is represented in Supplementary Table 5.
Figure 3 and Table 2 summarize subgroup analyses for CR per protocol variable.
Diet The CR was 82.5% (95% C.I. 78.7–86.5%, p < 0.001) among patients on clear liquids [1, 5, 12,13,14,15,16,17,18,19,20,21,22,23,24,25,26, 28, 32, 34,35,36,37,38, 40] (n = 3437) versus 83.6% (95% C.I. 71.5–97.8%, p < 0.001) in those allowed to ingest a low-fibre diet on the day before the procedure [14, 27, 29,30,31, 33, 39] (n = 635 patients).
Adjuvants CR was 80.8% (95% C.I. 74.2–87.9%, p < 0.001) among patients who received adjunctive laxatives [1, 5, 12,13,14,15,16, 22, 26, 28, 29, 31, 33, 36, 39] (n = 2224 patients), versus 84.4% (95% C.I. 80.1–88.9%, p < 0.001) when no adjuncts were associated before the purgative solution administration [12, 14, 17, 18, 20, 21, 23,24,25, 27, 30, 32, 34, 35, 37, 38, 40] (n = 1848).
Prokinetics CR was 75.7% (95% C.I. 64.0–89.5%, p = 0.132) when no prokinetic drugs were used [34, 36] (n = 258), versus 80.3% (95% C.I. 72.1–89.4%, p < 0.001) when its use was selectively only in those patients with delayed gastric passage of the capsule assessed real time during the examination [1, 5, 16, 22, 32, 35, 37,38,39,40] (n = 2408), and 84.4% (95% C.I. 79.9–89.2%, p < 0.001) when it was offered routinely to all patients before swallowing the capsule [12,13,14,15, 17,18,19,20,21, 23,24,25,26,27,28,29,30,31, 33] (n = 1406).
Purgative solution CR was 74.4% (95% C.I. 66.2–83.7%, p < 0.001) for PEG-Asc [13, 15, 23, 29, 30, 34, 38, 39] (n = 1068), 88.6% (95% C.I. 79.1–99.3%, p = 0.007) for < 4L PEG [14, 24, 27, 31, 33] (n = 138), and 86.0% (95% C.I. 82.2–90.1%, p < 0.001) when patients ingested the 4L PEG solution [1, 5, 12,13,14, 16, 17, 19,20,21,22, 25, 26, 28, 32, 35,36,37, 40] (n = 2866).
Booster CR was 86.2% (95% C.I. 82.3–90.2%, p < 0.001) for NaP [12,13,14, 17,18,19,20,21,22, 24,25,26,27,28, 30, 32, 34, 38, 40, 41] (n = 1908), 77.8% (95% C.I. 68.4–88.3%, p < 0.001) for PEG-based [12, 15, 23, 30, 31, 33] (n = 213 patients), 73.7% (95% C.I. 59.3–91.6%, p < 0.001) for OSS [1, 15, 16, 37, 39] (n = 1478), and 83.7% (95% C.I. 72.1–97.2%, p < 0.001) for gastrografin-based boosters [5, 15, 16, 29, 36] (n = 473).
The routine use of prokinetics prior to capsule ingestion [OR 1.86 (95% C.I. 1.13; 3.05), p = 0.015] and the use of split-dose PEG-only instead of the low-volume PEG-Asc as the purgative solution [OR 2.03 (1.01; 4.09), p = 0.048] were associated with significantly higher rate of complete examination—Fig. 4 and Table 3. In multivariate analysis, although no variable was associated with the outcome, a trend was observed for complete examinations when a prokinetic agent was used, either routinely, OR 1.80 (95% C.I. 0.72; 4.50; p = 0.21) or selectively, OR 2.12 (95% C.I. 0.98; 4.55; p = 0.055), as well as with the use of split-dose PEG as the purgative solution, versus the low-volume PEG-Asc, OR 1.78 (95% C.I. 0.88; 3.60; p = 0.109)—Table 4.
Safety and Tolerability
Data on the incidence of adverse events and tolerability of bowel preparation protocols were scarce and heterogeneous across the literature, with great variability among the different studies regarding definition, report, and classification. While the incidence of serious adverse events related to bowel preparation was exceedingly rare, the incidence of mild to moderate adverse events, such as nausea, vomiting, dizziness, vertigo, and/or abdominal pain, ranged from 0 [19, 21, 28, 33, 38, 40], to less than 10% [1, 13, 15, 22, 23, 34, 37, 39, 42], 10–30% [5, 25, 32, 35, 36, 42], > 30% [14, 24], or even higher incidence rates up to 80–90% being reported on a few studies [12, 27]—Supplementary Table 6. We did not find any significant associations between the incidence of adverse events and a particular type of bowel preparation.
Discussion
The quality of bowel preparation is fundamental in colonoscopy, influencing key outcomes such as the rate of complete examinations or the adenoma detection rate [43]. In the case of CC or PCE, achieving a clear mucosal view, as well as reaching a complete examination, is particularly challenging, as it cannot be optimized during the examination by manoeuvres such as washing, suction of debris, on-demand insufflation/deflation, or changing patients’ position. Our group has previously demonstrated that the risk of inadequate cleansing in CC is particularly high when there is history of inadequate colon cleansing, use of chronic laxative, antidepressant, or impaired mobility [44]. However, despite a recently published systematic review on this topic, there is still ongoing controversy on what should be the ideal bowel preparation protocol for CC or PCE [45]. In that study, the authors focused on the type of laxative, booster and prokinetic(s), concluding that both CRs and ACRs remain suboptimal, PEG laxative and NaP booster being the most commonly used, in spite of not associating with higher CRs or ACRs; in our current study, we further evaluated dietary factors, the use of adjunctive laxatives, and type of administration of the prokinetics (routine vs. selective). Moreover, we excluded mixed or less representative bowel preparation protocols and performed subgroup analysis per type and volume of laxative and per booster main component when mixed, with the purpose of decreasing heterogeneity for data analysis. Still, we acknowledge the significant heterogeneity of our data analysis, which was expected considering (i) the stepwise nature of the different bowel-cleansing protocols, (ii) the different timings for administration of the various components of the bowel preparation protocol, and (iii) the different criteria to evaluate the quality of bowel preparation. Nonetheless, our results suggest that the highest proportions of adequate bowel cleansing (ACR) were obtained using a low-fibre diet the day before the capsule examination, pre-medication with an adjunctive laxative such as sennosides, routine prokinetic prior to capsule ingestion, split dose < 4L PEG as the purgative solution, and a gastrografin-based booster. The highest proportion of complete examinations (CR) were observed using a low-fibre diet on the day before the capsule examination, with no use of adjunctive laxative, routine prokinetic administration prior to capsule ingestion, using a split dose < 4L PEG as the purgative solution, and NaP as the booster.
We should underline that although current evidence and international guidelines suggest that low-volume bowel preparations are valid options for bowel preparation in conventional colonoscopy, being equally effective and safe, and with improved tolerability when compared to classical large volume PEG preparations, this cannot be extrapolated to CC or PCE [46]. Indeed, our results demonstrate that low-volume PEG-Asc is significantly less effective for bowel cleansing and completeness in CC or PCE. Regarding the use of prokinetics (10 mg metoclopramide or 20 mg domperidone), our results suggest that they should be routinely administered to all patients before capsule ingestion; we can hypothesize that, similarly with what often occurs with the small bowel capsules protocols [47], a second prokinetic dose may be offered if the capsule passage to the small bowel is delayed > 1 h as assessed with the real-time viewer. Regarding the booster to administer after the capsule reaches the small bowel, NaP has been the most extensively evaluated and remains the most consistent alternative. Although NaP is currently not recommended as bowel preparation for conventional colonoscopy, mainly due to safety issues related to hyperosmolarity and nephrotoxicity, those have not been observed in CC or PCE studies, even in at-risk frail populations [48]. The use of gastrografin as booster warrants further evaluation, following some promising initial results. Although protocols using gastrografin-based boosters have been associated with improved outcomes, the magnitude of the effect attributable to gastrografin is difficult to extrapolate, since in every study, gastrografin was associated with some type of supplementary booster. A recently published prospective cohort study using historical controls described that adding gastrografin to the standard sodium phosphate booster protocol improved the rate of adequate bowel preparation, although further work is needed to increase the complete CC rate [49].
Further expected refinements to improve the outcomes in terms of mucosal visibility may include the addition of simethicone (100–200 mg) to the bowel preparation protocol, as it has been consistently shown to significantly improve the quality of bowel preparation and is currently recommended in conventional colonoscopy studies, by reducing the number of bubbles covering the mucosal surface [46, 50]. However, it should be noted that there is no standardized protocol in terms of dose or timing of administration in CC or PCE studies.
Finally, from the literature review, it is well established that patients should receive a 10 mg bisacodyl suppository as a “rescue laxative” 1–2 h after the second booster if the capsule had not been excreted, and a light snack may be allowed following the bisacodyl suppository administration.
Regarding tolerability and safety, there were not enough organized data to allow for quantitative or qualitative analysis. However, we could observe that all the different protocols were generally safe, with no severe adverse events reported and being reasonably well tolerated by the vast majority of patients.
In summary, our results suggest that, similarly to what is currently recommended for conventional colonoscopy [46], a low-fibre diet could be allowed the day before the procedure, as it does not impair the quality of cleansing or the completion rate and improves patients’ tolerability and adherence to the bowel preparation protocol; PEG to be used as the purgative solution administered in split dose, at a volume of less than 4L (mainly 3L), which was superior to 4L regarding completeness and adequate cleansing rates; prokinetics (10 mg metoclopramide or 20 mg domperidone) were more effective towards completeness when routinely offered to all patients before capsule ingestion; routine use of a sennoside laxative prior to the purgative solution effectively improved cleansing quality, while the addition of simethicone or the use of composite boosters, such as an association of NaP and gastrografin, mainly in populations with higher risk for inadequate bowel preparation [38], seem promising but require further evaluation.
A major strength of this systematic review and meta-analysis was the inclusion of a large number of patients from prospective studies with good methodological quality. The main limitation derives from the heterogeneity between studies, namely the composite multi-step bowel preparation protocols and uneven methodologies at evaluating the main efficacy and safety outcomes, and an effort towards standardization of bowel preparation protocols and outcomes assessment tools are warranted.
In conclusion, the results of this systematic review and meta-analysis were able to consolidate the current evidence on the core components of the standard bowel preparation protocols for CC and PCE, while identifying some key aspects for improvement of cleansing quality and completeness, as the main efficacy outcomes to be validated in further well-designed studies.
Abbreviations
- ACR:
-
Rate of adequate cleansing
- CR:
-
Rate of complete examinations
- PCE:
-
Pan-intestinal capsule endoscopy
- PEG:
-
Polyethylene glycol
- PEG-Asc:
-
Polyethylene glycol-ascorbate
- NaP:
-
Sodium phosphate
- CC:
-
Colon capsule
- OSS:
-
Oral sulphate solution
References
Rex DK, Adler SN, Aisenberg J et al. Accuracy of capsule colonoscopy in detecting colorectal polyps in a screening population. Gastroenterology. 2015;148:948-957.e942.
Eliakim R, Spada C, Lapidus A et al. Evaluation of a new pan-enteric video capsule endoscopy system in patients with suspected or established inflammatory bowel disease - feasibility study. Endosc Int Open. 2018;6:E1235–E1246.
Cortegoso Valdivia P, Elosua A, Houdeville C et al. Clinical feasibility of panintestinal (or panenteric) capsule endoscopy: a systematic review. Eur J Gastroenterol Hepatol. 2021;33:949–955.
Mascarenhas-Saraiva M. Is capsule colonoscopy the solution for incomplete conventional colonoscopy? Rev Esp Enferm Dig. 2017;109:319–321.
Spada C, Hassan C, Barbaro B et al. Colon capsule versus CT colonography in patients with incomplete colonoscopy: a prospective, comparative trial. Gut. 2015;64:272–281.
Carvalho PB, Rosa B, Cotter J. Mucosal healing in Crohn’s disease - are we reaching as far as possible with capsule endoscopy? J Crohns Colitis. 2014;8:1566–1567.
Moher D, Shamseer L, Clarke M et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1.
Leighton JA, Rex DK. A grading scale to evaluate colon cleansing for the PillCam COLON capsule: a reliability study. Endoscopy. 2011;43:123–127.
Rokkas T, Papaxoinis K, Triantafyllou K, Pistiolas D, Ladas SD. Does purgative preparation influence the diagnostic yield of small bowel video capsule endoscopy?: a meta-analysis. Am J Gastroenterol. 2009;104:219–227.
Yung DE, Rondonotti E, Sykes C, Pennazio M, Plevris JN, Koulaouzidis A. Systematic review and meta-analysis: is bowel preparation still necessary in small bowel capsule endoscopy? Expert Rev Gastroenterol Hepatol. 2017;11:979–993.
Debray TP, Moons KG, Abo-Zaid GM, Koffijberg H, Riley RD. Individual participant data meta-analysis for a binary outcome: one-stage or two-stage? PLoS ONE. 2013;8:e60650.
Spada C, Riccioni ME, Hassan C, Petruzziello L, Cesaro P, Costamagna G. PillCam colon capsule endoscopy: a prospective, randomized trial comparing two regimens of preparation. J Clin Gastroenterol. 2011;45:119–124.
Argueelles-Arias F, San-Juan-Acosta M, Belda A et al. Preparations for colon capsule endoscopy. Prospective and randomized comparative study between two preparations for colon capsule endoscopy: PEG 2 liters + ascorbic acid versus PEG 4 liters. Revista Espanola De Enfermedades Digestivas. 2014;106:312–317.
Ramos L, Alarcon O, Adrian Z et al. One-day versus two-day cleansing for colon capsule endoscopy: a prospective randomized pilot study. Gastroenterol Hepatol. 2014;37:101–106.
Kroijer R, Dyrvig AK, Kobaek-Larsen M, Stovring JO, Qvist N, Baatrup G. Booster medication to achieve capsule excretion in colon capsule endoscopy: a randomized controlled trial of three regimens. Endosc Int Open. 2018;6:E1363-e1368.
Kastenberg D, Burch WC Jr, Romeo DP et al. Multicenter, randomized study to optimize bowel preparation for colon capsule endoscopy. World J Gastroenterol. 2017;23:8615–8625.
Schoofs N, Deviere J, Van Gossum A. PillCam colon capsule endoscopy compared with colonoscopy for colorectal tumor diagnosis: a prospective pilot study. Endoscopy. 2006;38:971–977.
Van Gossum A, Munoz-Navas M, Fernandez-Urien I et al. Capsule endoscopy versus colonoscopy for the detection of polyps and cancer. N Engl J Med. 2009;361:264–270.
Gay G, Delvaux M, Frederic M, Fassler I. Could the colonic capsule PillCam Colon be clinically useful for selecting patients who deserve a complete colonoscopy?: results of clinical comparison with colonoscopy in the perspective of colorectal cancer screening. Am J Gastroenterol. 2010;105:1076–1086.
Pilz JB, Portmann S, Peter S, Beglinger C, Degen L. Colon capsule endoscopy compared to conventional colonoscopy under routine screening conditions. BMC Gastroenterol. 2010;10:66.
Herrerias-Gutierrez JM, Argueelles-Arias F, Caunedo-Alvarez A et al. PillCam (c) colon capsule for the study of colonic pathology in clinical practice. Study of agreement with colonoscopy. Revista Espanola De Enfermedades Digestivas. 2011;103:69–75.
Spada C, Hassan C, Munoz-Navas M et al. Second-generation colon capsule endoscopy compared with colonoscopy. Gastrointest Endosc. 2011;74:581–589.
Hartmann D, Keuchel M, Philipper M et al. A pilot study evaluating a new low-volume colon cleansing procedure for capsule colonoscopy. Endoscopy. 2012;44:482–486.
Alarcon-Fernandez O, Ramos L, Adrian-de-Ganzo Z et al. Effects of colon capsule endoscopy on medical decision making in patients with incomplete colonoscopies. Clin Gastroenterol Hepatol. 2013;11:534-540.e531.
Sung J, Ho KY, Chiu HM, Ching J, Travis S, Peled R. The use of Pillcam Colon in assessing mucosal inflammation in ulcerative colitis: a multicenter study. Endoscopy. 2012;44:754–758.
Remes-Troche JM, Jimenez-Garcia VA, Garcia-Montes JM, Hergueta-Delgado P, Roesch-Dietlen F, Herrerias-Gutierrez JM. Application of colon capsule endoscopy (CCE) to evaluate the whole gastrointestinal tract: a comparative study of single-camera and dual-camera analysis. Clin Exp Gastroenterol. 2013;6:185–192.
Ye CA, Gao YJ, Ge ZZ et al. PillCam colon capsule endoscopy versus conventional colonoscopy for the detection of severity and extent of ulcerative colitis. J Dig Dis. 2013;14:117–124.
San Juan-Acosta M, Caunedo-Álvarez A, Argüelles-Arias F et al. Colon capsule endoscopy is a safe and useful tool to assess disease parameters in patients with ulcerative colitis. Eur J Gastroenterol Hepatol. 2014;26:894–901.
Togashi K, Fujita T, Utano K et al. Gastrografin as an alternative booster to sodium phosphate in colon capsule endoscopy: safety and efficacy pilot study. Endosc Int Open. 2015;3:E659–E661.
Alvarez-Urturi C, Fernandez-Esparrach G, Ibanez IA et al. Accuracy of colon capsule endoscopy in detecting colorectal polyps in individuals with familial colorectal cancer: could we avoid colonoscopies? Gastroenterol Res Pract. 2017;2017:1507914.
Igawa A, Oka S, Tanaka S, Otani I, Kunihara S, Chayama K. Evaluation for the clinical efficacy of colon capsule endoscopy in the detection of laterally spreading. Tumors Dig. 2017;95:43–48.
Shi HY, Chan FKL, Higashimori A et al. A prospective study on second-generation colon capsule endoscopy to detect mucosal lesions and disease activity in ulcerative colitis (with video). Gastrointest Endosc. 2017;86:1139-1146 e1136.
Zhou J, Tang X, Wang J, Chen Z, Wang X, Jiang B. Feasibility of a novel low-volume and sodium phosphate-free bowel preparation regimen for colon capsule endoscopy. Exp Ther Med. 2017;14:1739–1743.
Baltes P, Bota M, Albert J et al. PillCamColon2 after incomplete colonoscopy—a prospective multicenter study. World J Gastroenterol. 2018;24:3556–3566.
Voska M, Zavoral M, Grega T et al. Accuracy of colon capsule endoscopy for colorectal neoplasia detection in individuals referred for a screening colonoscopy. Gastroenterol Res Pract. 2019;2019:5975438.
Pecere S, Senore C, Hassan C et al. Accuracy of colon capsule endoscopy for advanced neoplasia. Gastrointest Endosc. 2020;91:406-414.e401.
Bruining DH, Oliva S, Fleisher MR, Fischer M, Fletcher JG, BLINK study group. Panenteric capsule endoscopy versus ileocolonoscopy plus magnetic resonance enterography in Crohn’s disease: a multicentre, prospective study. BMJ Open Gastroenterol. 2020;7:e000365.
de Sousa Magalhaes R, Boal Carvalho P, Rosa B, Moreira MJ, Cotter J. The prediction of inadequate colon capsule cleansing: a cohort selection guided by CC-CLEAR GE. Port J Gastroenterol. 2022;29:311–321.
Vuik FER, Moen S, Nieuwenburg SAV, Schreuders EH, Kuipers EJ, Spaander MCW. Applicability of colon capsule endoscopy as pan-endoscopy: from bowel preparation, transit, and rating times to completion rate and patient acceptance. Endosc Int Open. 2021;9:E1852–E1859.
Eliakim R, Yassin K, Niv Y et al. Prospective multicenter performance evaluation of the second-generation colon capsule compared with colonoscopy. Endoscopy. 2009;41:1026–1031.
Voska M, Suchanek S, Majek O et al. The efficiency of colonic capsule endoscopy in detection of colorectal polyps and cancers comparing to colonoscopy: multicenter, prospective crosses over study. United Eur Gastroenterol J. 2013;1:A493.
Kastenberg D, Burch WC Jr, Romeo D, Kashyap PK, Pound D, Rex DK. A multicenter, consecutive, randomized study to optimize the bowel preparation regimen for colon capsule endoscopy. Am J Gastroenterol. 2016;111:S178–S179.
Kaminski MF, Thomas-Gibson S, Bugajski M et al. Performance measures for lower gastrointestinal endoscopy: a European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative. Endoscopy. 2017;49:378–397.
de Sousa Magalhaes R, Arieira C, Boal Carvalho P, Rosa B, Moreira MJ, Cotter J. Colon Capsule CLEansing Assessment and Report (CC-CLEAR): a new approach for evaluation of the quality of bowel preparation in capsule colonoscopy. Gastrointest Endosc. 2021;93:212–223.
Bjoersum-Meyer T, Skonieczna-Zydecka K, Cortegoso Valdivia P et al. Efficacy of bowel preparation regimens for colon capsule endoscopy: a systematic review and meta-analysis. Endosc Int Open. 2021;9:E1658–E1673.
Hassan C, East J, Radaelli F et al. Bowel preparation for colonoscopy: European Society of Gastrointestinal Endoscopy (ESGE) guideline—update 2019. Endoscopy. 2019;51:775–794.
Cotter J, de Castro FD, Magalhaes J, Moreira MJ, Rosa B. Finding the solution for incomplete small bowel capsule endoscopy. World J Gastrointest Endosc. 2013;5:595–599.
Carretero C, Prieto de Frias C, Angos R et al. Pan-enteric capsule for bleeding high-risk patients. Can we limit endoscopies? Rev Esp Enferm Dig. 2021;113:580–584.
Macleod C, Oliphant R, Richards C, Watson AJM. An evaluation of a novel bowel preparation regimen and its effect on the utility of colon capsule endoscopy: a prospective cohort study with historical controls. Tech Coloproctol. 2022;27:665–672.
Liu X, Yuan M, Li Z, Fei S, Zhao G. The efficacy of simethicone with polyethylene glycol for bowel preparation: a systematic review and meta-analysis. J Clin Gastroenterol. 2021;55(6):e46–e55.
Acknowledgments
Not applicable.
Funding
Open access funding provided by FCT|FCCN (b-on). This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
BR and JC conceived the study rationale and conceptual design. The manuscript was drafted by BR and revised by TCG, HD, and JC. BR, TCG, and HD contributed to the development of the selection criteria, risk of bias assessment strategy, and data extraction criteria. HD developed the search strategy and managed the study records. BR and TCG screened the studies obtained in the search for eligibility criteria, extracted data, and assessed the risk of bias of included studies. BS-P guided and critically revised all the statistical methods, analysis, data extraction, and interpretation. JC resolved all disagreements regarding eligibility for inclusion, data extraction, and/or risk of bias, and critically revised the manuscript. All authors have read and approved the final manuscript. BR is the guarantor of this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The protocol for this systematic review has been registered with the International Prospective Register of Systematic Reviews by the Centre for Reviews and Dissemination at the University of York (PROSPERO), with the ID: CRD42020176645.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Rosa, B., Donato, H., Cúrdia Gonçalves, T. et al. What Is the Optimal Bowel Preparation for Capsule Colonoscopy and Pan-intestinal Capsule Endoscopy? A Systematic Review and Meta-Analysis. Dig Dis Sci 68, 4418–4431 (2023). https://doi.org/10.1007/s10620-023-08133-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-023-08133-7