Abstract
Background
The rs641738 C > T single-nucleotide polymorphism of MBOAT7 has been associated with hepatocellular carcinoma (HCC) and nonalcoholic fatty liver disease (NAFLD). Latin Americans have high rates of HCC and NAFLD, but no assessment between MBOAT7 and HCC has been performed in this population.
Aims
We provide the first assessment of the impact of MBOAT7 on HCC risk in Latin Americans.
Methods
Patients were prospectively recruited into the ESCALON network, designed to collect samples from Latin American patients with HCC in 6 South American countries (Argentina, Ecuador, Brazil, Chile, Peru, and Colombia). A European cohort and the general Hispanic population of gnomAD database were included for comparison. Associations between HCC and MBOAT7 were evaluated using logistic regression.
Results
In total, 310 cases of HCC and 493 cases of cirrhosis without HCC were assessed. The MBOAT7 TT genotype was not predictive of HCC in Latin Americans (TT vs CC OR adjusted = 1.15, 95% CI 0.66–2.01, p = 0.610) or Europeans (TT vs CC OR adjusted = 1.20, 95% CI 0.59–2.43, p = 0.621). No significant association was noted on subgroup analysis for NAFLD, viral hepatitis, or alcohol-related liver disease. The TT genotype was increased in the NAFLD-cirrhosis cohort of Latin Americans compared to a non-cirrhotic NAFLD cohort (TT vs CC + CT OR = 2.75, 95% CI 1.10–6.87, p = 0.031).
Conclusion
The rs631738 C > T allele of MBOAT7 was not associated with increased risk of HCC in Latin Americans or Europeans. An increase in the risk of cirrhosis was noted with the TT genotype in Latin Americans with NAFLD.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the third most common cause of cancer-associated death worldwide [1,2,3]. Much of the increased rate of HCC is driven by increased rates of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) with NAFLD prevalence now reaching 30% globally [4]. Latin America has some of the highest rates of NAFLD in the world and has seen a recent drastic increase in the proportion of HCC cases secondary to NAFLD [5,6,7,8,9]. In comparison to other liver diseases, NAFLD has higher rates of HCC in non-cirrhotic individuals who would not meet current clinical practice guidelines for HCC screening [10,11,12,13]. Latin Americans with HCC diagnosed through surveillance have better outcomes, but the majority are actually diagnosed outside of surveillance [14].
Genetics play an important role in HCC occurrence and genetic susceptibility assessment has the potential to improve HCC risk stratification [15]. Genome-wide studies (GWAS) have identified numerous single-nucleotide polymorphisms (SNP) associated with a variety of liver pathologies, including HCC [16]. The rs641738 C > T gene polymorphism of MBOAT7, which is associated with decreased MBOAT7 expression and increased hepatic fat accumulation, has been associated with the development of HCC in chronic liver conditions, including NAFLD [17,18,19,20,21]. However, the association has been inconsistent which may be due to different effects of the allele on different underlying liver pathologies and on different ethnicities. Indeed, the great majority of studies assessing MBOAT7 in HCC have been performed in European or Asian cohorts and do not include Latin American populations, effectively limiting the use of this SNP in a population with much need of NAFLD-related HCC risk stratification. Interestingly, multiple studies have associated the MBOAT7 T allele (the variant allele) with HCC development in Europeans with NAFLD, but the association has not been consistently replicated in Asian populations with NAFLD or European populations with other etiologies of liver disease [17, 20,21,22,23,24,25,26,27,28].
In this study we aimed, for the first time, to address the association between the presence of MBOAT7 rs641738 C > T SNP and HCC development in a Latin American population through evaluation of samples of the ESCALON network (comprised of 6 countries in South America) to further understand the role of this SNP in risk stratification for HCC in this population. We included a population of patients from the Netherlands to allow for comparisons between HCC patients across Latin America and Europe in a direct fashion and to evaluate the impact ethnicity may have on MBOAT7’s influence on HCC development.
Methods
Samples and Study Subjects
This is a cross-sectional study which evaluated patients recruited into the ESCALON network (www.escalon.eu). The network was established in 2018 and is composed of a total of seven medical centers from six South American countries as well as one medical center from the Netherlands which provided the European participants [29]. Recruitment of patients into the network was based on availability and was not randomized. Patients were classified as “Latin American” or “European” based on geographic location and not self-identified ethnicity. Patient information and blood samples were collected at each individual institution and were registered in a Research Electronic Data Capture registry (REDCap). All patient data were de-identified and required to include previous informed consent with ethics approval in all centers. Patients were classified as having HCC based on biopsy or American Association for the Study of Liver disease established imaging criteria. Patients were classified as having alcohol-related liver disease if they had persistent steatohepatitis in the setting of prolonged ethanol intake, defined as 30 g/day for women and 40 g/day for men over 10 years. The diagnosis of NAFLD was determined by the managing hepatologist or by evidence of hepatic steatosis on pathology or imaging in the absence of other clear reasons for hepatic steatosis. Individuals without viral hepatitis, NAFLD, or alcohol-related liver disease were categorized as “other” etiology, which encompassed both known causes and unknown causes. Patients were determined to have a mixed etiology of liver disease if they had any combination of hepatitis B virus (HBV), hepatitis C virus (HCV), NAFLD, and alcohol-related liver disease. These patients were not included in subgroup analysis. All data was regularly checked by a data monitor, and double checked by the managing site prior to the analysis of the data.
Genotyping
For single-nucleotide polymorphism testing, genomic DNA was extracted from whole blood (Gentra Puregene), according to the manufacturer’s protocol. The rs641738 C > T MBOAT7 SNP was genotyped using TaqMan probe pre-designed SNP genotyping assays (Thermo Fisher). Genotyping was performed using StepOne-Plus Real-Time PCR System and a Custom TaqMan SNP Genotyping Assay (Applied Biosystems). qPCR were carried out in a 10-μl reaction volume containing 4 µl of genomic DNA, 6 µl genotyping master mix with probe.
Statistical Analysis
Demographic and clinical characteristics were compared between different subjects with continuous variables presented as means with standard deviations and categorical variables displayed as proportions. The Student’s t test was used to compare continuous variables and chi-square was used to compare proportions. Univariable and multivariable logistical regression were used to assess for factors associated with HCC, including the presence of rs641738 C > T MBOAT7. Patients without cirrhosis (including those with HCC) were excluded from the assessment of HCC risk. Likewise, patients with HCC were excluded from the assessment of cirrhosis risk. Odds ratios and 95% confidence intervals were presented for each variable. SAS Software Version 9.4 (The SAS Institute, Cary, NC) was utilized for statistical computations.
Results
Patient Characteristics
A total of 310 cases of HCC and 493 cases of cirrhosis without HCC were included in analysis. The median age of the combined cohort was 65 years (IQR 59–70 years). The median age was 65 years (IQR 59–71 years) in Latin Americans and 64 years (IQR 56–70 years) in Europeans. For those with HCC, median ages were 68 years (IQR 62–73 years) and 67 years (IQR 62–71 years) in Latin Americans and Europeans, respectively. The Latin American-combined HCC and cirrhosis cohort was composed of 45.1% women and the European-combined HCC and cirrhosis cohort was composed of 28.6% women (p < 0.001). NAFLD was the most common etiology of liver disease in Latin Americans with and without HCC accounting for 50% of cases (Table 1). In comparison, the combined HCC and non-HCC European population had a lower proportion of NAFLD cases (20%) and increased proportions of alcohol use (25%) and HCV (25%).
MBOAT7 HCC Risk Assessment
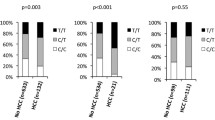
A variety of inheritance patterns were considered when evaluating for an association between the T variant allele and HCC and the following comparisons were made: T vs C, TT vs CC, TT vs CT + CC, TT + CT vs CC, and CT vs CC. Latin Americans with HCC had higher rates of the risk allele TT genotype compared to Latin Americans without HCC (19% and 16%, respectively, Fig. 1), but this difference was not statistically significant (p = 0.364). Europeans with HCC had higher rates of TT genotype (21%) compared to those without HCC (16%), but this difference was also not significant (p = 0.387). No statistically significant difference was noted in the CC genotype and CT genotype frequencies between those with and without HCC in either cohort (Table 2). The TT genotype was not predictive of HCC in either cohort but trended toward a non-statistically significant increased risk in both cohorts with adjusted ORs for TT vs CC of 1.15 and 1.20 in Latin Americans and Europeans, respectively. In a multivariable logistic regression model that included MBOAT7, advanced age was predictive of HCC in the Latin American cohort and diabetes was predictive of HCC in Europeans (Table 3). Minor allele frequency (MAF) was evaluated within the gnomAD database, a genome aggregation database with 17,720 sequences belonging to Latin Americans (https://gnomad.broadinstitute.org/) [30]. The MAF within the gnomAD database was 33.0% in Latin Americans. We found the MAF of our Latin American with HCC cohort to be 40.8% (OR = 1.40, 95% CI 1.12–1.75, p = 0.003). Notably, this did not differ significantly from the MAF in our cohort of Latin Americans with cirrhosis without HCC (OR = 1.03, 95% CI 0.79–1.36, p = 0.817).
The genotype frequency distribution of the MBOAT7 rs641738 C > T single-nucleotide polymorphism in patients with hepatocellular carcinoma compared to patients with cirrhosis without hepatocellular carcinoma. Genotype frequency is stratified by geographic patient location in two separate cohorts, Europeans and Latin Americans. HCC hepatocellular carcinoma
In subgroup analysis based on underlying liver disease (Table 4), the TT genotype was not associated with HCC in Latin Americans with NAFLD (TT vs CC OR adjusted = 1.07, 95% CI 0.51–2.29, p = 0.846). No significant association was found between the TT genotype and HCC in Latin Americans with viral hepatitis or alcohol-related liver disease. In the European cohort, a non-statistically significant increase in the odds of HCC was noted for NAFLD, viral hepatitis, and alcohol-related liver disease with the most prominent increase being in viral hepatitis (OR adjusted = 2.04, 95% CI 0.55–7.56, p = 0.284).
Effect of Ancestry in HCC Risk Related to MBOAT7
The majority (84%) of Latin Americans with HCC or cirrhosis evaluated in this study were of non-European descent. MAF for Latin Americans of non-European descent was 40.6% and in those of European descent was 38.8% (p = 0.667), suggesting that rates of the SNP are similar regardless of ancestry in Latin Americans. Genotype distribution did not significantly differ based on ancestry in Latin Americans. When restricting analysis to just those with HCC, the genotype distribution between Latin Americans of European and non-Europeans descent remained similar (Fig. 2). Importantly, the genotype distribution for Europeans with HCC did not significantly differ from Latin Americans of European descent (TT 20% vs 18%, Online Resource 1).
The genotype frequency distribution of the MBOAT7 rs641738 C > T single-nucleotide polymorphism in patients with hepatocellular carcinoma. Frequencies are compared between patients of different current geographic location and descent with a European cohort, a Latin American cohort of European descent and a Latin American cohort of non-European descent
Effect of MBOAT7 in Cirrhosis
To assess if MBOAT7 influenced cirrhosis development, a separate cohort of Latin American and European patients with chronic liver disease without HCC or cirrhosis was included. Of the 112 European patients included, HBV was the cause of liver disease in 60% (67/112) of patients. In comparison, HBV represented the cause of liver disease in only 8% (12/157) of Europeans with cirrhosis. Given the substantial difference in etiologies of liver disease between the European patients with and without cirrhosis, no assessment of the impact of MBOAT7 on cirrhosis development was made in this group of European patients. The Latin American chronic liver disease cohort, which was also not randomly recruited, displayed a high proportion of cases due to NAFLD. There were 94 Latin Americans in the cohort with 87% having NAFLD and 41% of this group being females. The median ages of those with and without cirrhosis were 64 years (IQR 59–70 years) and 59 years (IQR 50–65 years), respectively. Mean body mass index (BMI) was increased in the group with cirrhosis (30 vs 27 kg/m2, p < 0.001) as was the rate of diabetes (35% vs 10%, p < 0.001). We found a significant difference of the TT genotype frequency between patients with NAFLD-cirrhosis (17.8%) and NAFLD without cirrhosis (7.3%) (OR TT vs CT + CC = 2.75, 95% CI 1.10–6.87, p = 0.031). MAF in the NAFLD-cirrhosis cohort was 42.2% compared to 33% in the Latino population in gnomAD (OR = 1.48, 95% CI 1.20–1.84, p < 0.001).
Discussion
We found no statistically significant association between the MBOAT7 rs641738 C > T SNP and HCC in a Latin American population. To our knowledge, this is the first assessment of the effect of the MBOAT7 SNP on HCC development in patients from this region of the world. We also evaluated a European cohort for comparison and similarly found no association between the SNP and HCC development.
The MBOAT7 protein is an enzyme that catalyzes acyl-chain remodeling of phospholipids within Land’s cycle which subsequently creates membrane diversity within the fatty composition of the inner leaflet of cell membranes [31,32,33]. The rs641738 C > T SNP of MBOAT7 is associated with decreased hepatic expression of MBOAT7 [22, 34], which has been associated with liver steatosis and increased triglyceride synthesis [22, 35,36,37,38]. Moreover, a recent study suggested that MBOAT7 is a negative regulator of toll-like receptors (TLR) and decreased expression of MBOAT7 subsequently results in increased activation of TLRs and therefore increased inflammation which could lead to increased risk for HCC [37, 39].
Previous studies evaluating the association between MBOAT7 and HCC have been inconsistent (Online Resource 2). Donati et al. found a significant association between the allele and HCC in a mixed alcohol-related liver disease and HCV European cohort and Wang et al. found a small statistically significant association in an Asian cohort of patients with HBV [17, 27]. However, consistent with our results, numerous others have found no association between MBOAT7 and HCC in alcohol-related liver disease or viral hepatitis [17, 22, 23, 25, 26]. Donati et al. also found the allele to be associated with HCC in a European cohort of patients with NAFLD (OR per allele = 1.65, p = 0.021) [17]. Pelusi et al. subsequently found an association between MAF and HCC in a discovery cohort, but not in the validation cohort of patients with NAFLD and Bianco et al. found no association between allele carrier status and HCC in yet another European cohort with NAFLD [21, 28]. No association between MAF and HCC in an Asian population with NAFLD was found when assessed by Kawaguchi et al. [24]. We provided the first assessment in Latin Americans with NAFLD and similarly did not find a significant association.
Numerous possible reasons exist for why no association was found between the SNP and HCC in Latin Americans in our study. The SNP may truly not be associated with an increased risk in this population or the allele may be associated with HCC only in certain etiologies of liver disease and the limited sample sizes of our subgroups prevented detection of the association. It may also be possible that the MBOAT7 SNP is associated with non-cirrhotic HCC. Although individuals with HCC without cirrhosis were excluded from the analysis, we genotyped 16 Latin Americans with HCC without cirrhosis. Of the 16, one had the TT genotype (risk allele), eight were heterozygotes, and seven were CC homozygotes.
The MBOAT7 rs641738 C > T SNP has been associated with NAFLD development and severity in European populations, but the association has not been present when evaluating Asian populations [40,41,42,43]. This suggests a potential different effect MBOAT7 may have on the full spectrum of liver disease depending on ethnicity. While MBOAT7 rs641738 C > T and the development of HCC had not been studied in Latin Americans prior to this study, the effects of the variant on NAFLD development and severity in Argentinians were assessed by Sookoian et al. [44]. No association between the variant and NAFLD or NASH development and severity was found by the group. Additionally, the variant was not associated with presence of fibrosis or lobular inflammation. Of note, all individuals in that study self-reported being of European ancestry, while our study includes mainly non-European descent. In contrast to these findings, we did find an increased proportion of the risk allele in patients with NAFLD-cirrhosis compared to those with NAFLD without cirrhosis in Latin Americans.
Several limitations of this study warrant discussion. No direct comparison with healthy controls was included. However, we assessed with overall Latino population in the gnomAD database which, in contrast to our results, did show a significant association between the allele and both HCC and cirrhosis. The sample size did not allow for evaluation of individuals with HCC without cirrhosis. The scope of assessed outcome was limited in this study as only presence or absence of HCC was assessed and important clinical factors such as disease extent and mortality were not. However, no other study to our knowledge has addressed this SNP in a large cohort of Latin Americans with HCC and cirrhosis.
In summary, we found no association between the MBOAT7 rs641738 C > T SNP and HCC in Latin American and European cohorts regardless of the combination of inheritance patterns. However, we did find an association between the SNP and presence of cirrhosis in NAFLD in Latin Americans.
Data availability
The data supporting the findings of this study are available from the corresponding author, JD, upon reasonable request.
References
Bray F, Ferlay J, Soerjomataram I et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. https://doi.org/10.3322/caac.21492.
Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J Hepatol. 2020;72:250–261.
World Health Organization. Number of new cases and deaths from liver cancer predicted to rise by more than 55% by 2040. (2022, October 6). Retrieved from https://www.iarc.who.int/wp-content/uploads/2022/10/pr320_E.pdf.
Younossi ZM, Golabi P, Paik JM et al. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77:1335–1347. https://doi.org/10.1097/HEP.0000000000000004.
Dongiovanni P, Stender S, Pietrelli A et al. Causal relationship of hepatic fat with liver damage and insulin resistance in nonalcoholic fatty liver. J Intern Med. 2018;283:356–370. https://doi.org/10.1111/joim.12719.
Pinero F, Pages J, Marciano S et al. Fatty liver disease, an emerging etiology of hepatocellular carcinoma in Argentina. World J Hepatol. 2018;10:41–50.
Farah M, Anugwom C, Ferrer JD et al. Changing epidemiology of hepatocellular carcinoma in South America: A report from the South American liver research network. Ann Hepatol. 2023;28:100876. https://doi.org/10.1016/j.aohep.2022.100876.
Rojas YAO, Cuellar CLV, Barron KMA, Arab JP, Miranda AL. Non-alcoholic fatty liver disease prevalence in Latin America: A systematic review and meta-analysis. Ann Hepatol. 2022;27:100706. https://doi.org/10.1016/j.aohep.2022.100706.
Castellanos-Fernandez MI, Pal SC, Arrese M, Arab JP, George J, Mendez-Sanchez N. Nonalcoholic Fatty Liver Disease in Latin America and Australia. Clin Liver Dis. 2023;27:301–315. https://doi.org/10.1016/j.cld.2023.01.015.
Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18:223–238. https://doi.org/10.1038/s41575-020-00381-6.
Mittal S, El-Serag HB, Sada YH et al. Hepatocellular Carcinoma in the Absence of Cirrhosis in United States Veterans is Associated With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2016;14:124–31 e1. https://doi.org/10.1016/j.cgh.2015.07.019.
Reig M, Gambato M, Man NK et al. Should Patients With NAFLD/NASH Be Surveyed for HCC? Transplantation. 2019;103:39–44.
Llovet JM, Willoughby CE, Singal AG et al. Nonalcoholic steatohepatitis-related hepatocellular carcinoma: pathogenesis and treatment. Nat Rev Gastroenterol Hepatol. 2023. https://doi.org/10.1038/s41575-023-00754-7.
Debes JD, Chan AJ, Balderramo D et al. Hepatocellular carcinoma in South America: Evaluation of risk factors, demographics and therapy. Liver Int. 2018;38:136–143. https://doi.org/10.1111/liv.13502.
Yang Y, Wu QJ, Xie L et al. Prospective cohort studies of association between family history of liver cancer and risk of liver cancer. Int J Cancer. 2014;135:1605–1614. https://doi.org/10.1002/ijc.28792.
Buch S, Stickel F, Trepo E et al. A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis. Nat Genet. 2015;47:1443–1448. https://doi.org/10.1038/ng.3417.
Donati B, Dongiovanni P, Romeo S et al. MBOAT7 rs641738 variant and hepatocellular carcinoma in non-cirrhotic individuals. Sci Rep. 2017;7:4492. https://doi.org/10.1038/s41598-017-04991-0.
Carlsson B, Linden D, Brolen G et al. Review article: the emerging role of genetics in precision medicine for patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2020;51:1305–1320. https://doi.org/10.1111/apt.15738.
Bianco C, Casirati E, Malvestiti F et al. Genetic predisposition similarities between NASH and ASH: Identification of new therapeutic targets. JHEP Rep. 2021;3:100284. https://doi.org/10.1016/j.jhepr.2021.100284.
Teo K, Abeysekera KWM, Adams L et al. rs641738C>T near MBOAT7 is associated with liver fat, ALT and fibrosis in NAFLD: A meta-analysis. J Hepatol. 2021;74:20–30. https://doi.org/10.1016/j.jhep.2020.08.027.
Pelusi S, Baselli G, Pietrelli A et al. Rare Pathogenic Variants Predispose to Hepatocellular Carcinoma in Nonalcoholic Fatty Liver Disease. Sci Rep. 2019;9:3682. https://doi.org/10.1038/s41598-019-39998-2.
Thabet K, Asimakopoulos A, Shojaei M et al. MBOAT7 rs641738 increases risk of liver inflammation and transition to fibrosis in chronic hepatitis C. Nat Commun. 2016;7:12757. https://doi.org/10.1038/ncomms12757.
Degasperi E, Galmozzi E, Pelusi S et al. Hepatic Fat-Genetic Risk Score Predicts Hepatocellular Carcinoma in Patients With Cirrhotic HCV Treated With DAAs. Hepatology. 2020;72:1912–1923. https://doi.org/10.1002/hep.31500.
Kawaguchi T, Shima T, Mizuno M et al. Risk estimation model for nonalcoholic fatty liver disease in the Japanese using multiple genetic markers. PLoS One. 2018;13:e0185490. https://doi.org/10.1371/journal.pone.0185490.
Stickel F, Buch S, Nischalke HD et al. Genetic variants in PNPLA3 and TM6SF2 predispose to the development of hepatocellular carcinoma in individuals with alcohol-related cirrhosis. Am J Gastroenterol. 2018;113:1475–1483. https://doi.org/10.1038/s41395-018-0041-8.
Raksayot M, Chuaypen N, Khlaiphuengsin A et al. Independent and additive effects of PNPLA3 and TM6SF2 polymorphisms on the development of non-B, non-C hepatocellular carcinoma. J Gastroenterol. 2019;54:427–436. https://doi.org/10.1007/s00535-018-01533-x.
Wang P, Li Y, Li L, Zhong R, Shen N. MBOAT7-TMC4 rs641738 Is Not Associated With the Risk of Hepatocellular Carcinoma or Persistent Hepatitis B Infection. Front Oncol. 2021;11:639438. https://doi.org/10.3389/fonc.2021.639438.
Bianco C, Jamialahmadi O, Pelusi S et al. Non-invasive stratification of hepatocellular carcinoma risk in non-alcoholic fatty liver using polygenic risk scores. J Hepatol. 2021;74:775–782. https://doi.org/10.1016/j.jhep.2020.11.024.
Debes JD, Boonstra A, Balderramo D et al. Hepatobiliary cancers in South America: disparity strikes. Lancet Gastroenterol Hepatol. 2019;4:581.
Chen S, Francioli LC, Goodrich JK et al. A genome-wide mutational constraint map quantified from variation in 76,156 human genomes. bioRxiv. 2022. https://doi.org/10.1101/2022.03.20.485034.
Romeo S, Sanyal A, Valenti L. Leveraging Human Genetics to Identify Potential New Treatments for Fatty Liver Disease. Cell Metab. 2020;31:35–45.
Varadharajan V, Massey WJ, Brown JM. Membrane-bound O-acyltransferase 7 (MBOAT7)-driven phosphatidylinositol remodeling in advanced liver disease. J Lipid Res. 2022;63:100234. https://doi.org/10.1016/j.jlr.2022.100234.
Caddeo A, Hedfalk K, Romeo S, Pingitore P. LPIAT1/MBOAT7 contains a catalytic dyad transferring polyunsaturated fatty acids to lysophosphatidylinositol. Biochim Biophys Acta Mol Cell Biol Lipids. 2021;1866:158891. https://doi.org/10.1016/j.bbalip.2021.158891.
Meroni M, Longo M, Fracanzani AL, Dongiovanni P. MBOAT7 down-regulation by genetic and environmental factors predisposes to MAFLD. EBioMedicine. 2020;57:102866. https://doi.org/10.1016/j.ebiom.2020.102866.
Meroni M, Longo M, Tria G, Dongiovanni P. Genetics Is of the Essence to Face NAFLD. Biomedicines. 2021;9:1359. https://doi.org/10.3390/biomedicines9101359.
Tanaka Y, Shimanaka Y, Caddeo A et al. LPIAT1/MBOAT7 depletion increases triglyceride synthesis fueled by high phosphatidylinositol turnover. Gut. 2021;70:180–193. https://doi.org/10.1136/gutjnl-2020-320646.
Zhang Y, Guo T, Yang F et al. Single-nucleotide rs738409 polymorphisms in the PNPLA3 gene are strongly associated with alcoholic liver disease in Han Chinese males. Hepatol Int. 2018;12:429–437. https://doi.org/10.1007/s12072-018-9889-3.
Krawczyk M, Rau M, Schattenberg JM et al. Combined effects of the PNPLA3 rs738409, TM6SF2 rs58542926, and MBOAT7 rs641738 variants on NAFLD severity: a multicenter biopsy-based study. J Lipid Res. 2017;58:247–255. https://doi.org/10.1194/jlr.P067454.
Rico Montanari N, Anugwom CM, Boonstra A, Debes JD. The Role of Cytokines in the Different Stages of Hepatocellular Carcinoma. Cancers (Basel). 2021;13:4876. https://doi.org/10.3390/cancers13194876.
Alharthi J, Bayoumi A, Thabet K et al. A metabolic associated fatty liver disease risk variant in MBOAT7 regulates toll like receptor induced outcomes. Nat Commun. 2022;13:7430. https://doi.org/10.1038/s41467-022-35158-9.
Ismaiel A, Dumitrascu DL. Genetic predisposition in metabolic-dysfunction-associated fatty liver disease and cardiovascular outcomes-Systematic review. Eur J Clin Invest. 2020;50:e13331. https://doi.org/10.1111/eci.13331.
Xia Y, Huang CX, Li GY et al. Meta-analysis of the association between MBOAT7 rs641738, TM6SF2 rs58542926 and nonalcoholic fatty liver disease susceptibility. Clin Res Hepatol Gastroenterol. 2019;43:533–541. https://doi.org/10.1016/j.clinre.2019.01.008.
Koo BK, Joo SK, Kim D et al. Development and Validation of a Scoring System, Based on Genetic and Clinical Factors, to Determine Risk of Steatohepatitis in Asian Patients with Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2020;18:2592–9 e10. https://doi.org/10.1016/j.cgh.2020.02.011.
Sookoian S, Flichman D, Garaycoechea ME et al. Lack of evidence supporting a role of TMC4-rs641738 missense variant-MBOAT7- intergenic downstream variant-in the Susceptibility to Nonalcoholic Fatty Liver Disease. Sci Rep. 2018;8:5097. https://doi.org/10.1038/s41598-018-23453-9.
Acknowledgments
European-Latin American ESCALON consortium, funded by the EU Horizon 2020 program, project number 825510, the Foundation for Liver and Gastrointestinal Research (SLO), AIRP UMN and NIH-R21TW012390-01A1.
Funding
The authors have no funding support to disclose outside of those noted in the acknowledgment of grant support. The funding sources for this work were not involved in the design of the study, data collection and analysis, writing of the report, or the decision to submit for publication.
Author information
Authors and Affiliations
Contributions
All authors contributed to the Study design. Material preparation, data collection, and analysis were performed by SG, JA, ZMAG, JO, JP, DB, JDF, AZM, MA, and EC. AB and JDD had the original conception, supervised the study, and are the warrantors of the manuscript. The first draft of the manuscript was written by SG and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The contributing authors have no financial, professional, personal, or other conflicts of interest to declare.
Ethical approval
Each participating organization provided ethical approval of the study and all study procedures. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments.
Informed consent
Written informed consent for each patient in their natural language was obtained from all individuals prior to participation in the ESCALON study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Goble, S., Akambase, J., Prieto, J. et al. MBOAT7 rs641738 Variant Is Not Associated with an Increased Risk of Hepatocellular Carcinoma in a Latin American Cohort. Dig Dis Sci 68, 4212–4220 (2023). https://doi.org/10.1007/s10620-023-08104-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-023-08104-y