Abstract
Background
Mast cell activation syndrome (MCAS) is a clinically heterogeneous disease with allergy-like symptoms and abdominal complaints. Its etiology is only partially understood and it is often overlooked.
Aims
The aim of this study was to identify subgroups of MCAS patients to facilitate diagnosis and allow a personalized therapy.
Methods
Based on data from 250 MCAS patients, hierarchical and two-step cluster analyses as well as association analyses were performed. The data used included data from a MCAS checklist asking about symptoms and triggers and a set of diagnostically relevant laboratory parameters.
Results
Using a two-step cluster analysis, MCAS patients could be divided into three clusters. Physical trigger factors were particularly decisive for the classification as they showed remarkable differences between the three clusters. Cluster 1, labeled high responders, showed high values for the triggers heat and cold, whereas cluster 2, labeled intermediate responders, presented with high values for the trigger heat and low values for cold. The third cluster, labeled low responders, did not react to thermal triggers. The first two clusters showed more divers clinical symptoms especially with regard to dermatological and cardiological complaints. Subsequent association analyses revealed relationships between triggers and clinical complaints: Abdominal discomfort is mainly triggered by histamine consumption, dermatological discomfort by exercise, and neurological symptoms are related to physical exertion and periods of starvation. The reasons for the occurrence of cardiological complaints are manifold and triggers for respiratory complaints still need better identification.
Conclusion
Our study identified three distinct clusters on the basis of physical triggers, which also differ significantly in their clinical symptoms. A trigger-related classification can be helpful in clinical practice for diagnosis and therapy. Longitudinal studies should be conducted to further understand the relationship between triggers and symptoms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mast cell activation syndrome (MCAS) is characterized by allergy-like symptoms as a result of pathologically increased activation of mast cells and is sometimes associated with accumulation of mast cells of abnormal morphology [1]. MCAS-associated symptoms are caused by downstream effects of mast cell mediators, which mast cells release upon activation and which normally play a role in the defense against harmful substances or microorganisms, such as bacteria, parasites, and animal toxins [2, 3]. One important mast cell mediator is histamine, which increases permeability and dilatation of vessels, resulting in swelling and redness [4]. Mast cells are most known for their involvement in type I allergy, in which IgE binds tightly to the mast cell receptor FcεRI and, therefore, induces mast cell activation upon antigen contact [5]. MCAS is clinically indistinguishable from systemic mastocytosis (SM), a rare genetic disease typically caused by mutations in the KIT gene coding for the KIT tyrosine-protein kinase. Both diseases are characterized by increased mast cell activation and accumulation of mast cells in a variety of tissues [6]. The symptoms of MCAS and SM patients are very diverse and include abdominal, neurological, cardiological, respiratory, and dermatological complaints [7,8,9,10,11]. The severity of symptoms ranges from mild over severe to life-threatening [12, 13]. Characteristically, at the beginning of the manifestation of the disease, symptoms occur episodically, and a progression of symptoms is observed during the course of the disease [14].
For a lot of patients, abdominal complaints are the major problem in their disease. These complaints have many similarities with those in irritable bowel syndrome (IBS). IBS is classically characterized by chronic diffuse abdominal pain, constipation, and diarrhea. Studies have shown that refractory IBS patients present with symptoms typical of MCAS and have elevated mast cell-specific laboratory parameters indicative of mast cell activation [15]. It was also found that IBS patients on ketotifen therapy, an anti-histamine, had a significant reduction in symptoms [16]. Based on these similarities, it appears likely that there is an overlap of the two disorders, highlighting the importance of clear diagnostic criteria and more research about etiology and pathology of both disorders.
MCAS is in real life severely underdiagnosed despite an estimated prevalence of up to 17% [17], with negative consequences for affected patients and the healthcare system. In an effort toward the definition of clear diagnostic criteria for MCAS, two main approaches (termed Consensus 1 and Consensus 2) have emerged [6, 12, 18,19,20,21,22,23,24]. The most recent versions of Consensus 1 [20] and 2 [24] assign different criteria as a basis for the diagnosis of MCAS (Table 1). As has been discussed recently [6], the criteria for diagnosis of Consensus 1 are slightly stricter than those of Consensus 2, whereas, at this point in time, it is debatable which approach is more accurate.
Regardless of stricter or more lenient criteria, both approaches have the goal to improve MCAS diagnosis. Many MCAS patients have not yet been identified as such, are unaware of their disease, and therefore do not benefit from mast cell-specific therapy or even receive inappropriate therapies.
Various medications are used to treat MCAS. These include anti-histamines [25], the mast cell stabilizer cromoglycic acid [26], and slow-release vitamin C [27], which all reduce mast cell activity and can reduce symptoms. In cases of treatment failure, immunosuppressants or omalizumab [28] can be tried [1]. A facultative symptomatic treatment is necessary to break the vicious cycle of continuously mutually activating mast cells. Prerequisite for a response to every drug therapy is the avoidance of mast cell trigger factors [10, 12].
As mentioned above, MCAS is a disorder with variable clinical presentation [6]. Accordingly, symptoms, triggers, and laboratory values vary widely among patients. Against this background and the estimated high prevalence [14], it is evident that a more precise characterization of specific subgroups could facilitate diagnosis and therapy. Cluster analysis is an explorative statistical procedure in which subgroups, i.e., clusters, are formed on the basis of various characteristics. Cluster analyses have already proven to be beneficial in the characterization of other clinical diseases, such as fibromyalgia [29] and irritable bowel syndrome [30]. We therefore conducted a cluster analysis of MCAS patients based on symptoms and triggers, with the aim of simplifying the classification of patients on the basis of their medical history and open the road toward a more personalized therapy.
Methods
Participants
Data of 250 MCAS patients were included in the study either from the specialized private practice of one of the authors (MM) or from the Center for Rare Diseases at Bonn University Hospital, between January 2019 and June 2020. Requirements for inclusion in the study were an age of at least 18 years and a proven mast cell activation syndrome according to consensus 2 criteria [6]. Patients completed the checklist as part of the diagnostic procedure. In this process, a conspicuous score in the checklist represented one criterion for the diagnosis of MCAS. Only patients in whom the diagnosis was subsequently confirmed by the minor criteria abnormal biopsy result and/or abnormalities in mast cell-specific laboratory parameters were included in this study.
Instruments and Collected Data
The previously published checklist [14, 21] for the detection of mast cell mediator release syndrome [14] served as the data basis. In this checklist, patients were asked in a binary fashion for various abdominal, neurological, cardiological, dermatological, and respiratory symptoms as well as certain factors leading to elicitation or exacerbation of symptoms [14]. The initial trigger of the complaints could be named in a free text column. In addition, data on age, sex, weight, and height were collected. Mast cell-specific laboratory parameters such as tryptase in blood and N-methylhistamine in urine had been assessed for more than 180 patients by MVZ Labor Quade, Cologne, Germany. Chromogranin A, neuron-specific enolase, and immunoglobulin E levels had also been determined for the vast majority of MCAS patients [31,32,33,34].
Statistical Analysis
Cluster Analysis
Two-step [35] and hierarchical [36] cluster analyses were performed using SPSS version 27.0 for macOS from IBM SPSS, Chicago IL, USA. Factors that trigger discomfort or lead to worsening of discomfort turned out to lead to separable clusters and were therefore considered for cluster analysis. These included physical exertion, heat, cold, stress, alcohol consumption, sleep deprivation, periods of starvation, and consumption of histamine-containing foods. Bayesian information criterion (BIC) was used to determine the optimal number of clusters. Cluster solutions were compared with the silhouette measure for cohesion and separation. In the second step, the three identified clusters were compared in terms of baseline data collected, symptoms, and laboratory values using cross-tabulations. Significant differences in the subgroups were detected using the Chi-square test for categorical and the Kruskal–Wallis test for continuous variables. p-values of ≤ 0.01 were considered significant.
Clinical Associations
Possible associations between sex, triggers, symptoms, and laboratory values were calculated. For two binary variables, the p-value was calculated by Fisher’s exact test. When comparing a binary variable with a numerical one, the p-value for the Wilcoxon–Mann–Whitney test was calculated. In this study, due to a wide variety of questions and the exploratory nature of the study, many tests for significance were performed. It should therefore be noted that with a significance level of p ≤ 0.01, there is an average of 1 × erroneous rejection of the null hypothesis in 100 tests performed.
Results
Description of the Collective
In the MCAS patient cohort, about 86% were female. The average patient age was 45 years (range 18–84 years). The mean weight of the patients was within the normal range with a BMI of 23.2 kg/m2 (range 12.4–46.2 kg/m2).
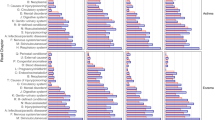
Triggers of symptoms varied widely, with stress and consumption of histamine-containing foods reported most frequently (Fig. 1A). Heat was named as a trigger factor more often than cold. The initial trigger could be identified by almost half of the patients and was mainly related to stress, surgery or hospitalization, and infections (Fig. 1B).
A The prevalence of the triggers in the study cohort is given as a percentage. B The prevalence of initial triggers is given as a percentage. C Result of two-step cluster analysis. A value of 1 means that 100 percent of the patients in the cluster reported this trigger, whereas a value of 0 indicates that none of the patients reported it. D Prevalence of the symptoms in the three clusters given as a percentage. p-values were calculated with chi-square test
The symptoms reported by the MCAS patients were very variable and affected different organ systems, including various abdominal, neurological, respiratory, cardiological, and dermatological symptoms. Neurological complaints, in particular word-finding disorder and weakness/exhaustion, affected almost every patient in the collective (Fig. 2A). On the other hand, other symptoms, in particular dermatological complaints, affected smaller subgroups (Fig. 2B–E). In the majority of the collective, symptoms occurred episodically and many observed a shortening of symptom-free intervals (Fig. 2F).
Laboratory chemistry showed an increase of the mean and median value only for N-methylhistamine. Tryptase, immunoglobulin E, chromogranin A, and neuron-specific enolase showed abnormal values only in a few patients (Fig. 4A–E).
Two-Step Cluster Analysis Worked Better than Hierarchical Cluster Analysis
To identify clusters, hierarchical cluster analyses were performed first. Although the resulting clusters showed already great similarity with the final results, they were not completely stable with regard to cluster size and values for the respective triggers in response to altered data sorting. Therefore, a two-step cluster analysis for binary data was conducted, since it is typically more robust with regard to sorting dependency [37]. However, using the two-step cluster analysis and taking all factors into account also shows a slightly varying result with different sorting of the data. For this reason, a predictor importance table supplied with the two-step cluster analysis was generated. This showed with descending influence physical exertion, heat, and cold as the three most important provoking factors (Fig. 3A). The other triggers showed a significant decrease in importance and therefore were not chosen as cluster variables, but were presented via cross-tabulations. Setting physical exertion, heat, and cold as variables and log-likelihood as a measure of quality, a robust clustering result was shown, which remained unchanged with different sorting of the data.
A Predictor importance table created with SPSS two-step cluster analysis. The formation of the clusters should be limited to the most important factors [47]. In this example, these could be clearly identified as physical exertion, heat, and cold. B Chart created with SPSS two-step cluster analysis, BIC values against number of clusters. The largest change in BIC values marks the optimal number of clusters. The largest change in BIC values was seen in the step from a 1 cluster to a 2 cluster and in the change from a 2-cluster to a 3-cluster solution. C Silhouette measure of cohesion and separation for a 2-cluster solution, the larger the silhouette measure for cohesion and separation becomes, the better the cluster result. D Silhouette measure of cohesion and separation for a 3-cluster solution
Bayesian information criterion (BIC) was used to determine the optimal number of clusters. The largest changes in the values were seen when jumping from a 1- to a 2-cluster solution and from a 2- to a 3-cluster solution. At 4 clusters and beyond, the curve flattened and the BIC values changed only slightly (Fig. 3B). The decision for a 3-cluster solution was made due to the better silhouette measure for cohesion and separation compared to the 2-cluster solution (Fig. 3C–D). In addition, the 3-cluster solution provided the best content interpretability (not shown).
The results showed that MCAS patients could be classified into three groups based on the factors that provoke symptoms or cause an exacerbation of existing symptoms (Fig. 1C). These factors include physical exertion, heat, cold, stress, alcohol consumption, insomnia, periods of starvation, and histamine consumption.
Cluster 1 Responded to All Triggers
The largest cluster with a total of 105 patients showed high scores on all triggers (Fig. 1C). Discomfort was particularly triggered by physical exertion, heat, and cold; the three triggers were reported by all patients of cluster 1. Less important was sleep deprivation and alcohol consumption, although these triggers were still mentioned by at least 70 percent of the patients of cluster 1 (Fig. 1C).
Cluster 2 Did Not React to Cold
In the smallest cluster (54 patients), cold was not a trigger of discomfort (Fig. 1C). Main triggers were instead physical exertion and heat which were reported by all patients of the cluster (Fig. 1C). The response to the other provoking factors was similar to that of the third cluster, but slightly more pronounced. Alcohol consumption and starvation periods were the least mentioned provoking factors (Fig. 1C).
Cluster 3 Showed Little Response to Physical Factors
The third cluster, consisting of 91 patients, showed the least response to the mentioned triggers (Fig. 1C). In contrast to the first two clusters, physical exertion and temperature extremes were not significant factors (Fig. 1C). Except for stress, the other triggers mentioned above were reported less by this group of patients than by those in clusters 2 and especially 1 (Fig. 1C).
Clinically, Clusters 1 and 2 Were More Severely Affected than Cluster 3
In the second step, the prevalence of the symptoms in the three clusters was compared (Fig. 1D). Patients from clusters 1 and 2 reported abdominal, cardiac, dermatologic, respiratory, and neurologic symptoms significantly more frequently than those of the third cluster (Fig. 1D). This was most pronounced for cardiological and dermatological symptoms. Increases in blood pressure, for example, were reported approximately twice as often by the first two clusters than by the third, and acne-like skin changes also occurred more than twice as frequently in the first two clusters. The first and the second cluster, on the other hand, did not differ significantly with regard to reported symptoms (Fig. 1D). Differences between these two patient groups (however, not reaching the level of significance) may exist with regard to neurological complaints and may reach statistical significance in future studies with larger patient numbers.
Laboratory Chemistry Results Between the Clusters
Laboratory chemistry revealed no evidence for significant differences between the three clusters with respect to the laboratory parameters immunoglobulin E, N-methylhistamine, tryptase, neuron-specific enolase, and chromogranin A (results not shown).
Association Analysis of Symptoms and Triggers
As our analysis revealed clusters that differed with respect to symptom triggers, we next asked if different triggers are typically associated with specific symptoms or if certain symptoms typically occur together. Indeed, closer inspection showed that abdominal symptoms were primarily triggered by histamine consumption. In addition, they were associated with episodic symptom onset and symptom progression during the course of the disease (Table 2).
For respiratory symptoms, no trigger showed a clear significant association with multiple respiratory symptoms. However, respiratory complaints co-occurred with symptoms in several other systems of the body, particularly neurological symptoms, like fatigue attacks, headache, and word-finding disorders (Table 2).
Dermatological complaints were most often associated with the trigger physical exertion. In particular, the occurrence of flushing, itching, and acne-like skin lesions showed a significant relationship to physical activity. Skin and mucous membrane complaints occurred together with respiratory complaints, such as runny nose and irritable cough (Table 2).
The triggers of the cardiological complaints were diverse. These symptoms were associated with the occurrence of physical weakness and exhaustibility as well as fatigue attacks (Table 2).
Neurological complaints were mainly triggered by physical exertion and periods of hunger. Cold led to symptoms of weakness and fatigue as well as headaches. Heat played only a minor role with regard to this symptom group. Neurological complaints showed many associations with other symptoms, especially cardiac and respiratory symptoms (Table 2).
There was a significant association between gender and renal N-methylhistamine excretion. Women had significantly higher mean and median values than men. In both sexes, median and mean values were above the reference value of 6.5 μg/mmol/Cr/m2 Body Surface Area (Fig. 4F).
A to E Boxplots for the respective laboratory parameters were in the reference range. F Boxplots of NMH created separately for men and women. The difference in urine NMH concentration between men and women was statistically significant as indicated by the p-value calculated with Wilcoxon–Mann–Whitney Test. Laboratory chemistry revealed normal mean values and medians for tryptase, chromogranin A, neuron-specific enolase, and immunoglobulin E (A–E). N-methylhistamine (NMH) in urine was elevated (average of 9.7 μg/mmol/Cr/m2 Body Surface Area versus the reference value of < 6.5). 75% of MCAS patients had median NMH levels above the reference value (F). The median NMH excretion was significantly higher in women than in men (F)
All calculated p-values of associations between symptoms and triggers can be found in Supplemental Table 1.
Discussion
Cluster Analysis
The central result of the present study is the possibility to divide MCAS patients into clinical subgroups. Taken together, the MCAS patient collective presents with a uniform reaction to stress, consumption of histamine, alcohol consumption, insomnia, and periods of hunger. The crucial difference appears in the evaluation of heat and cold as trigger factors, which resulted in the emergence of three clusters:
-
Cluster 1 can be considered as the group of high responders; complaints were caused by many triggers, including temperature changes in either direction. The latter were reported by all patients of the cluster.
-
Cluster 2 comprises the intermediate responders, who stated heat as a trigger but not cold, in contrast to the first cluster.
-
Cluster 3 covers the low responders, who were particularly notable for their low response to the main triggers of the first two clusters.
Classification of patients into three clusters allows the division of MCAS patients on the basis of their medical history. Physical triggers such as heat and cold are well known from previous studies [38, 39]. Similar findings can be observed in SM patients [40]. From a methodological perspective, a cluster analysis on the basis of trigger factors has not yet been applied to MCAS patients. Our study confirms the usefulness of this approach in MCAS patients as it allows for the subtyping of patients based on the anamnesis regarding the two physical triggers heat and cold, which is not only clinically meaningful but also can serve as an easily accessible and therefore an economic way to gain an estimate of the patient’s needs. Patients who report heat and/or cold will usually have more clinical symptoms (Fig. 1D) and require closer medical care than patients who do not report either trigger. Clusters 1 and 2 typically show more symptoms overall, but especially many dermatological and cardiological symptoms, to which special attention should therefore be paid.
The commonly used laboratory parameters immunoglobulin E, N-methylhistamine, tryptase, neuron-specific enolase, and chromogranin A did not show any significant differences in the three clusters and therefore cannot serve as indicators for one of the clusters. This is in accordance with previous reports showing that laboratory values are highly diverse among patients and do not correlate with diseases severity or symptoms ([6] and references therein).
Our results highlight the relevance of trigger factors and underline once more that patients should be advised to observe and subsequently avoid their specific triggers, in addition to drug therapy [1]. While three distinct clusters of patients emerged from our analysis, it should however be noted that individual complaints vary greatly among patients overall.
This type of heterogeneity among patients poses particular challenges not only to diagnosis, but also therapy. The relatively new field of precision medicine attempts to extract information, often from large amounts of data, aiming at providing personalized therapies based on the precise understanding of individual or stratified differences among patients, in order to be able to deliver to each patient the best type of therapy at the optimal time point and dosage to maximize efficiency [41]. At the same time, it enables the identification of specific biomarkers for identification of the optimal therapy for an individual patient. Our study could be seen as a first step toward tackling this heterogeneity in MCAS, although much more data combined with machine or deep learning strategies would be needed to identify precise differences between patients. For such an approach, several omics data would ideally be needed, e.g., patient genomes, single-cell omics of mast cells, (gut) microbiomics, as well as longitudinal, high-resolution data on diet, laboratory parameters and symptoms, and potentially even health monitoring data from wearable or mobile sensors. Knowledge about, e.g., the exact changes in mast cell genetics or signaling could have direct implications on the best symptomatic therapy, like, for example, the choice between mast cell stabilizers, anti-histamines, anti-IgE antibodies, or suppression of mast cell development [42] or even suggest new types of interventions, including diets to optimize gut microbiota for MCAS patients. Indeed, a specialized sub-area of precision medicine is precision nutrition, which tries to optimize individualized nutritional advice based on large amounts of data and machine learning or deep learning approaches [43], which could prove particularly beneficial for MCAS patients in the future, as many suffer from food intolerances and are often looking for the right diet to manage their symptoms.
Association Analysis
With the help of association analyses, previously unknown relationships between triggers and symptoms could be established. To be more specific, this means for clinical practice that patients with certain complaints can be given recommendations for action to improve their quality of life. This can be illustrated by specific examples: A patient with neurological complaints could be informed about his symptoms being most likely associated with starvation periods and physical exertion. If abdominal complaints occur, a progression of complaints and an episodic course of symptoms can be expected.
It should be noted that this method is rarely used, but offers great potential. In psychiatric research, association analyses and networks are already used to work out which symptoms are central to a disorder and how strongly they are related [44, 45]. With this in mind, it also seems possible that inferences can be made on a mechanistic basis. Thus, certain symptoms could occur together because of the same activation pathway or mediator being responsible. This also requires a better link between clinical and basic research to connect clinical findings with research results at the cellular level [46].
Limitations
The applied symptom checklist currently has a binary scale level. A more differentiated assessment that gives estimates of symptom severity may facilitate the understanding of differences in clinical symptomatology.
Furthermore, patients’ symptoms and triggers may change over time, which cannot be assessed by our study design. Therefore, it might be more appropriate to see clusters as disease stages, which evolve over time. Longitudinal, circadian, and environmental changes are also potential confounders for the interpretation of laboratory parameters, which were determined at only one time point as part of the diagnostic process. In follow-up studies, longitudinal data, including the laboratory parameters, should therefore be collected under controlled conditions (e.g., all patients on a specific diet) and at specific time points during the study, which would furthermore allow the inclusion of more objective data to complement subjective patient-reported experience measures, like the symptom checklist.
Conclusion
This is the first cluster analysis performed in MCAS patients and thus the first approach to dissect the genetically highly heterogeneous disease into less clinically heterogeneous subgroups. Clustering can have an immediate impact on daily clinical practice, as different clusters of a disease could have different needs with regard to therapy and supportive care. In this case, our results show that clinicians should especially take note of the triggers reported by patients, as they point toward different symptom loads that should be expected, which could prompt them to adjust symptomatic therapy accordingly. This is important in order to avoid exacerbation of symptoms by a vicious cycle of constantly mutually activating hyperactive mast cells. The information could be helpful to identify at-risk patients more easily.
The association analyses are new in the sense that they establish connections between triggers and symptoms that have not been described before. This allows an even more differentiated approach to the patient. Depending on which complaints the patient describes, the trigger can be named, a statement can be made about accompanying complaints, and recommendations about measures to reduce complaints can be given.
In summary, our study confirms the utility of a cluster analytic approach and the potential of association analysis to improve the understanding of MCAS and to personalize the therapy. To validate the results of our study, prospective longitudinal studies should be performed in future.
Data Availability
The dataset analyzed and discussed is available from the corresponding author upon request.
References
Molderings GJ, Haenisch B, Brettner S, Homann J, Menzen M, Dumoulin FL et al. Pharmacological treatment options for mast cell activation disease. Naunyn Schmiedebergs Arch Pharmacol. 2016;389:671–694.
Piliponsky AM, Romani L. The contribution of mast cells to bacterial and fungal infection immunity. Immunol Rev. 2018;282:188–197.
Kim HS, Kawakami Y, Kasakura K, Kawakami T. Recent advances in mast cell activation and regulation. F1000Res 2020;9:F1000.
da Silva EZM, Jamur MC, Oliver C. Mast cell function: a new vision of an old cell. J Histochem Cytochem. 2014;62:698–738.
Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18:693–704.
Afrin LB, Ackerley MB, Bluestein LS, Brewer JH, Brook JB, Buchanan AD et al. Diagnosis of mast cell activation syndrome: a global “consensus-2.” Diagnosis (Berl). 2020;8:137–152.
Afrin LB, Pöhlau D, Raithel M, Haenisch B, Dumoulin FL, Homann J et al. Mast cell activation disease: an underappreciated cause of neurologic and psychiatric symptoms and diseases. Brain Behav Immun. 2015;50:314–321.
Kolck UW, Haenisch B, Molderings GJ. Cardiovascular symptoms in patients with systemic mast cell activation disease. Transl Res. 2016;174:23-32.e1.
Weinstock LB, Pace LA, Rezaie A, Afrin LB, Molderings GJ. Mast cell activation syndrome: a primer for the gastroenterologist. Dig Dis Sci. 2020;66:965–982.
Picard M, Giavina-Bianchi P, Mezzano V, Castells M. Expanding spectrum of mast cell activation disorders: monoclonal and idiopathic mast cell activation syndromes. Clin Ther. 2013;35:548–562.
Qureshi AA, Friedman AJ. A review of the dermatologic symptoms of idiopathic mast cell activation syndrome. J Drugs Dermatol. 2019;18:162–168.
Molderings GJ, Brettner S, Homann J, Afrin LB. Mast cell activation disease: a concise practical guide for diagnostic workup and therapeutic options. J Hematol Oncol. 2011;4:10.
Bonadonna P, Bonifacio M, Lombardo C, Zanotti R. Hymenoptera allergy and mast cell activation syndromes. Curr Allergy Asthma Rep. 2015;16:5.
Molderings G, Kolck U, Scheurlen C, Brüss M, Frieling T, Raithel M et al. Die systemische Mastzellerkrankung mit gastrointestinal betonter Symptomatik - eine Checkliste als Diagnoseinstrument. Dtsch med Wochenschr. 2006;131:2095–2100.
Frieling T, Meis K, Kolck U, Homann J, Hülsdonk A, Haars U et al. Evidence for mast cell activation in patients with therapy-resistant irritable bowel syndrome. Z Gastroenterol. 2011;49:191–194.
Klooker TK, Braak B, Koopman KE, Welting O, Wouters MM, van der Heide S et al. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut. 2010;59:1213–1221.
Molderings GJ, Haenisch B, Bogdanow M, Fimmers R, Nöthen MM. Familial occurrence of systemic mast cell activation disease. PLoS ONE. 2013;8:e76241.
Valent P, Akin C. Doctor, I think I am suffering from MCAS: Differential diagnosis and separating facts from fiction. J Allergy Clin Immunol Pract. 2019;7:1109–1114.
Valent P, Akin C, Arock M, Brockow K, Butterfield JH, Carter MC et al. Definitions, criteria and global classification of mast cell disorders with special reference to mast cell activation syndromes: a consensus proposal. Int Arch Allergy Immunol. 2012;157:215–225.
Valent P, Akin C, Bonadonna P, Hartmann K, Brockow K, Niedoszytko M et al. Proposed diagnostic algorithm for patients with suspected mast cell activation syndrome (MCAS). J Allergy Clin Immunol Pract. 2019;7:1125-1133.e1.
Afrin LB, Molderings GJ. A concise, practical guide to diagnostic assessment for mast cell activation disease. WJH. 2014;3:1.
Afrin LB. The presentation, diagnosis and treatment of mast cell activation syndrome : review article. Curr Allergy Clin Immunol. 2014;27:146–160.
Afrin LB, Self S, Menk J, Lazarchick J. Characterization of mast cell activation syndrome. Am J Med Sci. 2017;353:207–215.
Molderings GJ, Zienkiewicz T, Homann J, Menzen M, Afrin LB. Risk of solid cancer in patients with mast cell activation syndrome: results from Germany and USA. F1000Res. 2017;6:1889.
Nurmatov UB, Rhatigan E, Simons FER, Sheikh A. H1-antihistamines for primary mast cell activation syndromes: a systematic review. Allergy. 2015;70:1052–1061.
Advenier C. Ruff F [Cromoglycic acid (disodium cromoglycate) and inhibitors of mast cell degranulation]. Sem Hop 1984;60:659–664.
Hagel AF, Layritz CM, Hagel WH, Hagel HJ, Hagel E, Dauth W et al. Intravenous infusion of ascorbic acid decreases serum histamine concentrations in patients with allergic and non-allergic diseases. Naunyn Schmiedebergs Arch Pharmacol. 2013;386:789–793.
Kibsgaard L, Skjold T, Deleuran M, Vestergaard C. Omalizumab induced remission of idiopathic anaphylaxis in a patient suffering from indolent systemic mastocytosis. Acta Derm Venereol. 2014;94:363–364.
de Souza JB, Goffaux P, Julien N, Potvin S, Charest J, Marchand S. Fibromyalgia subgroups: profiling distinct subgroups using the Fibromyalgia Impact Questionnaire. A preliminary study. Rheumatol Int. 2009;29:509–515.
Windgassen S, Moss-Morris R, Goldsmith K, Chalder T. The importance of cluster analysis for enhancing clinical practice: an example from irritable bowel syndrome. J Ment Health. 2018;27:94–96.
Schwartz LB, Metcalfe DD, Miller JS, Earl H, Sullivan T. Tryptase levels as an indicator of mast-cell activation in systemic anaphylaxis and mastocytosis. N Engl J Med. 1987;316:1622–1626.
Valent P, Bonadonna P, Hartmann K, Broesby-Olsen S, Brockow K, Butterfield JH et al. Why the 20% + 2 tryptase formula is a diagnostic gold standard for severe systemic mast cell activation and mast cell activation syndrome. Int Arch Allergy Immunol. 2019;180:44–51.
Payne V, Kam PCA. Mast cell tryptase: a review of its physiology and clinical significance. Anaesthesia. 2004;59:695–703.
Valent P, Horny HP, Triggiani M, Arock M. Clinical and laboratory parameters of mast cell activation as basis for the formulation of diagnostic criteria. Int Arch Allergy Immunol. 2011;156:119–127.
Chiu T, Fang D, Chen J, Wang Y, Jeris C. A robust and scalable clustering algorithm for mixed type attributes in large database environment. In: Proceedings of the seventh ACM SIGKDD international conference on Knowledge discovery and data mining [Internet]. New York, NY, USA: Association for Computing Machinery; 2001 [cited 2022 Apr 9]. p. 263–8. (KDD ’01). Available from: https://doi.org/10.1145/502512.502549
Li T. A general model for clustering binary data. In: Proceedings of the eleventh ACM SIGKDD international conference on Knowledge discovery in data mining [Internet]. New York, NY, USA: Association for Computing Machinery; 2005 [cited 2022 Apr 20]. p. 188–97. (KDD ’05). Available from: https://doi.org/10.1145/1081870.1081894
Clustering Binary Data (should be avoided) [Internet]. 2020 [cited 2021 Oct 12]. Available from: https://www.ibm.com/support/pages/clustering-binary-data-should-be-avoided
Hamilton MJ, Hornick JL, Akin C, Castells MC, Greenberger NJ. Mast cell activation syndrome: a newly recognized disorder with systemic clinical manifestations. J Allergy Clin Immunol. 2011;128:147-152.e2.
Theoharides TC, Tsilioni I, Ren H. Recent advances in our understanding of mast cell activation – or should it be mast cell mediator disorders? Expert Rev Clin Immunol. 2019;15:639–656.
Castells M, Austen KF. Mastocytosis: mediator-related signs and symptoms. Int Arch Allergy Immunol. 2002;127:147–152.
Hartl D, de Luca V, Kostikova A, Laramie J, Kennedy S, Ferrero E et al. Translational precision medicine: an industry perspective. J Transl Med. 2021;19:245.
Valent P, Akin C, Hartmann K, Nilsson G, Reiter A, Hermine O et al. Mast cells as a unique hematopoietic lineage and cell system: from Paul Ehrlich’s visions to precision medicine concepts. Theranostics. 2020;10:10743–10768.
Kirk D, Catal C, Tekinerdogan B. Precision nutrition: a systematic literature review. Comput Biol Med. 2021;133:104365.
Airaksinen J, Gluschkoff K, Kivimäki M, Jokela M. Connectivity of depression symptoms before and after diagnosis of a chronic disease: a network analysis in the U.S. Health and Retirement Study. J Affect Disord. 2020;266:230–234.
Mullarkey MC, Marchetti I, Beevers CG. Using network analysis to identify central symptoms of adolescent depression. J Clin Child Adolesc Psychol. 2019;48:656–668.
Kahn K, Ryan G, Beckett M, Taylor S, Berrebi C, Cho M et al. Bridging the gap between basic science and clinical practice: a role for community clinicians. Implement Sci. 2011;6:34.
IBM Corporation. IBM: SPSS Modeler/ Predictor Importance [Internet]. Predictor Importance. 2021 [cited 2022 Mar 30]. Available from: https://prod.ibmdocs-production-dal-6099123ce774e592a519d7c33db8265e-0000.us-south.containers.appdomain.cloud/docs/en/spss-modeler/18.1.0?topic=SS3RA7_18.1.0/modeler_mainhelp_client_ddita/clementine/idh_common_predictor_importance.html
Funding
Open Access funding enabled and organized by Projekt DEAL. There was no funding for this study.
Author information
Authors and Affiliations
Contributions
Conceptualization: TH, RC, MM, and JS. Methodology: TH, FK, RC, and JS. Formal analysis: TH, FK, RC, and JS. Investigation: TH. Resources: TH and MM. Writing—original draft preparation: TH, GJM, RC, MM, and JS. Writing, reviewing, and editing of the manuscript: TH, GJM, RC, MM, and JS. Visualization: TH, FK, and JS. Supervision: FK, RC, MM, and JS. Project administration: RC, MM, and JS.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no financial or non-financial competing interests.
Ethical approval
The study was approved by the Ethics Committee of the Rheinische Friedrich-Wilhelms-Universität Bonn, Medical Faculty, on April 30th to May 2022.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
An editorial commenting on this article is available at https://doi.org/10.1007/s10620-023-07923-3.
Supplementary Information
Below is the link to the electronic supplementary material.
10620_2023_7921_MOESM1_ESM.xlsx
Supplementary file1 (XLSX 21 kb). In the first column and the top line, the gender, laboratory values, and all triggers and symptoms queried are listed in each case. The respective p-values from Fisher’s exact test for two binary variables, and Wilcoxon–Mann–Whitey test for a binary and a numerical one is shown. p-values ≤ 0.05 are not shown individually and are indicated with NA.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Häder, T., Molderings, G.J., Klawonn, F. et al. Cluster-Analytic Identification of Clinically Meaningful Subtypes in MCAS: The Relevance of Heat and Cold. Dig Dis Sci 68, 3400–3412 (2023). https://doi.org/10.1007/s10620-023-07921-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-023-07921-5