Abstract
Background
Ongoing symptoms in treated celiac disease (CD) are frequent and are commonly thought of as being due to infractions to a gluten-free diet (GFD) or complications.
Aims
To study the etiology and natural history of clinically relevant events (CREs) throughout follow-up and identify predictors thereof to guide follow-up.
Methods
CREs (symptoms/signs requiring diagnostic/therapeutic interventions) occurring in celiac patients between January-2000 and May-2021 were retrospectively collected between June and September 2021 and analysed.
Results
One-hundred-and-eighty-nine adult patients (133 F, age at diagnosis 36 ± 13 years, median follow-up 103 months, IQR 54–156) were enrolled. CREs were very common (88/189, 47%), but hardly due to poor GFD adherence (4%) or complications (2%). Interestingly, leading etiologies were functional gastrointestinal disorders (30%), reflux disease (18%) and micronutrient deficiencies (10%). Age at diagnosis ≥ 45 years (HR 1.68, 95%CI 1.05–2.69, p = 0.03) and classical pattern of CD (HR 1.63, 95%CI 1.04–2.54, p = 0.03) were predictors of CREs on a multivariable Cox model. At 5 years, 46% of classical patients ≥ 45 years old at diagnosis were event-free, while this was 62% for non-classical/silent ≥ 45 years, 60% for classical < 45 years, and 80% for non-classical/silent < 45 years.
Conclusions
CREs occurred in almost half of CD patients during follow-up, with functional disorders being very common. New follow-up strategies for adult CD may be developed based on age and clinical pattern at diagnosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Celiac disease (CD) is a chronic immune-mediated enteropathy triggered by dietary gluten in genetically predisposed individuals [1,2,3] with a prevalence of around 1% in the general population [1,2,3,4].
The clinical presentation of CD is very heterogeneous, ranging from an overt malabsorption syndrome, to mild intestinal/extra-intestinal symptoms, or to asymptomatic patients [1,2,3]. Positive endomysial (EmA)/tissue transglutaminase (tTA) antibodies and villous atrophy (VA) on correctly oriented duodenal biopsies are still recommended for the diagnosis of CD in adults in most countries [1,2,3], although great interest has been dedicated to the possibility of a biopsy-sparing approach also in adults [5, 6]. A strict lifelong gluten-free diet (GFD) is the cornerstone for treatment of CD, leading to complete resolution of symptoms/histological lesions in the vast majority of patients, and preventing long-term morbidity and mortality associated to CD [1,2,3,4, 7,8,9,10,11]. However, it has been reported that in up to 30% of patients symptoms and/or histological lesions may not resolve completely despite being on a GFD [12,13,14,15,16,17,18].
These clinical scenarios have a wide spectrum of etiologies, which may be related or unrelated to CD. Ongoing gluten ingestion (voluntary or inadvertent) has been reported to be the prevalent cause of persistent symptoms in CD. However, other etiologies may also be responsible, ranging from purely functional gastrointestinal disorders, to life-threatening malignant complications of CD [12,13,14,15,16,17,18]. Despite their wide clinical variability, these scenarios are very often grouped together under the umbrella term non-responsive CD (NRCD). The literature provides data on the underlying etiology of NRCD, with particular interest on refractory CD and malignant complications of CD, for which risk factors and natural history have also been delineated [11, 12, 19,20,21]. Conversely, very little is known on the natural history of symptoms and disorders unrelated to complicated CD occurring in celiac patients on a long-term GFD and how this may influence modalities for organizing the follow-up of these patients. Although major international guidelines suggest maintaining regular follow-up consultations for all adult celiac patients, there is no widespread consensus on timing and modalities for organizing it cost-effectively [1,2,3]. Therefore, the present study aims to: (1) evaluate occurrence and etiologies of symptoms leading to clinically relevant events (CREs), both related and unrelated to CD, in adult celiac patients on long-term follow-up; (2) identify any relevant predictors of CREs during follow-up; 3) suggest possible follow-up strategies in adult celiac patients.
Patients and Methods
Study Population and Setting
This is a single-centre retrospective study of adult patients (≥ 18 years old) with biopsy-proven CD on a long-term GFD and follow-up, which aims to describe the natural history and identify the etiologies and predictors of persistent/recurrent symptoms despite a GFD. Patients enrolled in this study all underwent follow-up duodenal biopsy, as part of our standard of care in the last 20 years [22].
Enrolment and Exclusion Criteria
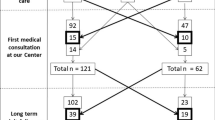
Patients who were directly diagnosed with CD at our center between January-2000 and November-2019, and followed-up in clinic until May-2021 were the focus of the present study. All patients underwent follow-up duodenal biopsy and regular dietary assessment of GFD adherence, either via interview by expert personnel or using a validated questionnaire we previously developed [23]. In the last 20 years our center has provided care to over 800 patients, as previously described [24]. To avoid biases, for the purpose of the present study, we included only patients directly diagnosed by our center who had at least one follow-up biopsy. In other words we excluded all referred patients and any patients who did not undergo follow-up duodenal biopsy for various reasons.
CD was diagnosed based on a certain degree of VA on duodenal biopsies from the second part of the duodenum and positive EmA and/or tTA while on a gluten-containing diet [1,2,3].
Data Collection
Patients’ medical records were retrospectively reviewed between June-2021 and September-2021, and the following data were collected: age at diagnosis of CD, presenting symptoms of CD according to Oslo classification [25], first-degree family history of CD, results and time of follow-up duodenal biopsies and EmA testing, number of follow-up medical consultations, duration of follow-up, GFD adherence throughout follow-up, and occurrence of clinical events, both related and unrelated to CD during follow-up.
Evaluation of Symptoms and Clinical Events During Follow-Up
Symptoms/signs (persistent, recurrent or new symptoms/signs) at any time during follow-up requiring any diagnostic testing, treatment, emergency room access, or hospitalization were considered CREs for the purpose of our study. Their etiologies and outcomes were also evaluated.
Persistent symptoms were defined as those already present at diagnosis of CD, which did not improve significantly or resolve during follow-up despite a GFD [22]; recurrent symptoms were those that relapsed despite initial resolution/improvement on a GFD; symptoms which developed for the first time while on a GFD, were considered ‘new onset symptoms’. Data on symptoms was collected regardless of whether a relationship with CD was suspected or not.
Diagnostic tests performed for scheduled follow-up of chronic conditions in the absence of symptoms, including follow-up of persistent VA, or in the context of a screening program were not considered as clinical events. This strategy was adopted in order to provide the most objective evaluation of CREs throughout follow-up in a retrospective study.
Clinical characteristics of patients with at least one event were compared to those of patients without events to identify predictors of developing events. Patients who had at least one clinically relevant event during follow-up as defined above were considered patients who developed events while those without any events were considered event-free. All events observed throughout follow-up were analysed to determine their aetiology and to estimate overall incidence of events.
Criteria to Assess GFD Adherence
All patients were instructed immediately after diagnosis of CD by expert personnel on how to follow a strict GFD correctly. Throughout follow-up, GFD adherence was assessed, until 2008, by dietary interview by expert personnel, after 2008 using a five-point validated score we previously developed [23, 24]. Patients scoring 0–2 were considered poorly adherent, while those scoring 3–4 were considered adherent.
Statistical Analysis
Statistical analysis was performed using R version 4.1.2 (R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/). Categorical variables were summarized as total counts and percentages. Univariate analysis of categorical variables was performed with Fisher’s exact test. The Cochran-Armitage test was used to evaluate trends in occurrence of events according to patient age groups. Continuous variables were summarized as mean and standard deviation or median and interquartile range. Testing for normality of data was performed using the Shapiro–Wilk test. For univariate analysis of continuous variables among groups the Mann–Whitney U test was used. Spearman rank correlation was used to evaluate for correlation among variables. Incidence rate of events and exact binomial 95% confidence intervals (95% CI) were calculated. Two-sided p-values < 0.05 were considered statistically significant. A multivariable Cox model was developed to identify predictors of event-free follow-up and to estimate hazard ratios and 95% CI. Harrel’s c statistic and 95% CI were calculated to evaluate model discriminatory ability. Variables for multivariable Cox model analysis were selected based on results of univariate analysis and on the basis of clinical relevance. Kaplan–Meier curves for event-free follow-up were generated and the logrank test was used to compare event-free follow-up rates among groups. Mortality among patients with and without events during follow-up was compared using the logrank test.
Results
One-hundred-and-eighty-nine celiac patients (133 F, mean age at diagnosis 36 ± 13 years) who underwent routine follow-up duodenal biopsy and were followed-up for a median of 112 months (IQR 73–164) were enrolled. At follow-up duodenal biopsy (after a median of 16 months since diagnosis, IQR 13–20 months), 29 patients (15.4%) still showed a certain degree of VA. Among these 29 patients, 2 (6.9%) had complicated CD, 9 (31.0%) had poor GFD adherence, while the remaining 18 (62.0%) showed initial but incomplete histological improvement in the context of good GFD adherence. GFD adherence at time of follow-up biopsy was good in the vast majority of patients (160/176, 90.9%, 13 data missing). Among patients without complications who underwent a further follow-up duodenal biopsy, histological recovery occurred in 75% of the patients while 25% still had atrophy due to poor GFD adherence.
At time of follow-up duodenal biopsy 95 patients overall (50.3%) had ongoing symptoms, of which 64/95 (37.0%) had persistence of symptoms present at diagnosis despite a GFD and 42/95 (24.6%) developed new symptoms which were not present at diagnosis. Only 11 of the 29 patients with VA at follow-up biopsy (37.9%) had persistent symptoms at time of follow-up biopsy.
Demographic and Clinical Characteristics of Patients With and Without Clinical Events
Eighty-eight out of 189 patients (47%) had at least one clinically significant event (symptoms requiring additional diagnostic testing, medical treatment, emergency room access or hospitalization) during follow-up, while the remaining 101 patients (53%) had no events. As shown in Table 1, patients who had at least one clinical event during follow-up were older at diagnosis (p = 0.02) and more likely to have a classical pattern of CD at presentation (p = 0.01). They were also more likely to have persistent VA at follow-up duodenal biopsy (p = 0.03). As shown in Fig. 1, there was a trend for increasing prevalence of events with increasing age at diagnosis (p < 0.01). No relationship was found between GFD adherence at time of follow-up duodenal biopsy and occurrence of events during follow-up (p = 0.44). In total, 5 patients died over follow-up, after a median of 146 months from diagnosis of CD (IQR 122–161). All patients that died had at least one clinical event during follow-up, while none of the patients who had no clinical events died during follow-up (mortality 5.7% vs 0%, p = 0.03). As would be expected, patients with clinical events had a significantly higher number of follow-up medical consultations in our Unit (median 4 vs 2, p < 0.01) and a slightly, although not statistically significant, longer duration of clinical follow-up than those with no events (median 125 vs 100 months, p = 0.06). Likewise, total number of events correlated with number of follow-up medical consultations (Spearman rank correlation, rho = 0.49, p < 0.001).
Clinical Events
Symptoms and Etiologies
In total 157 clinically significant events occurred throughout follow-up in all patients, resulting in an incidence of events of 83.5/1000 person-years (95% CI 70.9–97.6). Median time to event from diagnosis of CD was 50 months (IQR 26–107). Events, as shown in Fig. 2, were most prevalent in the first 5 years from diagnosis of CD and decreased thereafter (overall event-free follow-up rates were 65% at 5 years and 51% at 10 years). In managing the 157 events, which occurred, a total of 92 outpatient medical treatments, 63 diagnostic investigations and 13 emergency room or hospital admissions were required. The most common symptoms leading to clinical events were gastroesophageal reflux disease symptoms (30 cases, 19%), diarrhea (24, 15%), dyspepsia (21, 13%), and constipation (15, 10%). Figure 3 shows the underlying etiologies of clinical events the most common being gastroesophageal reflux disease (29, 18%) and functional gastrointestinal disorders (47, 30%) such as functional constipation (16, 10%), irritable bowel syndrome (15, 10%) and functional dyspepsia (14, 9%). Poor adherence to a GFD accounted for 4% of total events and complications of CD for only 2%.
Bar chart showing etiology of clinical events. GERD gastroesophageal reflux disease, GI gastrointestinal, GFD gluten-free diet CD coeliac disease, IBD inflammatory bowel disease, COPD chronic obstructive pulmonary disease. Functional GI disorders include: functional constipation, functional dyspepsia, irritable bowel syndrome, functional diarrhea. Two events which occurred in the context of poor GFD adherence (due to Helicobacter Pylori infection and dyslipidemia) were not included under poor GFD adherence as they were unrelated in nature to GFD adherence. Other etiologies included: colonic diverticular disease, colon polyps, dyslipidemia, dermatitis herpetiformis relapse, small intestinal bacterial overgrowth, COPD exacerbation, diabetic ketoacidosis in type 1 diabetes mellitus, acute gastroenteritis, ANCA-associated vasculitis, multifactorial anemia, hypercalcaemic syndrome, spondyloarthritis, osteoporosis, NSAID abuse, erosive gastritis, recurrent aphthous stomatitis
Diagnostic Tests
Of the 63 diagnostic tests performed, the most common ones were upper GI endoscopy with biopsies (28 cases, 44%), colonoscopy (13 cases, 21%) and abdominal ultrasonography (11 cases, 17%). The most common symptoms leading to diagnostic testing were diarrhoea (16 cases, 25%), dyspepsia (13 cases, 21%), abdominal pain (12 cases, 19%) and hematochezia (6 cases, 10%). Irritable bowel syndrome (12 cases, 19%), functional dyspepsia (10 cases, 16%), hemorrhoids (6 cases, 10%), gastroesophageal reflux disease/oesophagitis (5 cases, 8%) and lactose intolerance (4 cases, 6%) were the most common etiologies for symptoms requiring diagnostic investigations.
Need for Medical Treatment
Medical treatment was required in 92 events. The most common reasons for medical treatment were gastroesophageal reflux disease/oesophagitis (29 cases, 32%), micronutrient deficiencies (16 cases, 17%) including iron, folate and vitamin B12 deficiency with or without consequent anemia, functional constipation (15 cases, 16%), Helicobacter pylori infection (11 cases, 12%). Symptoms resolved after treatment in 75% of cases while they persisted in the remaining 25%, with functional constipation (43%), iron-deficiency anemia (21%), and H pylori infection (14%) being the most common to persist.
Emergency Room Access/Hospital Admission
Eight patients required emergency room/hospital admission during follow-up, with a total of 13 such events. Three patients were admitted to hospital due to persistence or relapse of severe malabsorption, all due to development of complications of CD (enteropathy-associated T-cell lymphoma, B-cell lymphoma, type 1 refractory CD). Two of these patients had primary unresponsiveness to a GFD (VA at follow-up biopsy and persistent malabsorption) while the last patient initially responded clinically and histologically to a GFD and after several years developed a complication [20]. Other reasons for emergency room access/hospital admission were not directly related to CD and included biliary colic, diabetic ketoacidosis and subsequent diagnosis of type 1 diabetes mellitus, acute gastroenteritis, ANCA-associated vasculitis, small intestinal bacterial overgrowth and exacerbation of chronic obstructive pulmonary disease (one patient each). Finally, one patient attended the emergency room and was subsequently hospitalized 4 times for reasons unrelated to CD (including hypercalcaemic syndrome, spondyloarthritis flare, multifactorial anemia). GFD adherence was good in all patients who attended the emergency room or were hospitalized.
Predictors of Clinical Events
Multivariable Cox analysis identified age at diagnosis ≥ 45 years (HR 1.68, 95%CI 1.05–2.69, p = 0.03) and classical pattern of CD at diagnosis (HR 1.63, 95% CI 1.04–2.54, p = 0.03) as independent predictors of developing clinical events during follow-up. However, persistence of VA at follow-up duodenal biopsy did not reach statistical significance (HR 1.55, 95%CI 0.91–2.63, p = 0.11). Harrell’s c was 0.60 (95%CI 0.54–0.66).
Distribution of Clinical Events During Follow-Up According to Age and Clinical Pattern at Diagnosis of CD
As shown in Fig. 4 occurrence of events during follow-up differs significantly (p < 0.01) according to age group (< 45 years, ≥ 45 years) and clinical pattern of CD at diagnosis (classical vs non-classical/silent). Overall, at 5 years follow-up, 46% of classical patients diagnosed at age ≥ 45 years were event-free, while event-free rates were 62% for non-classical/silent patients ≥ 45 years at diagnosis, 60% for classical patients < 45 years old at diagnosis, and 80% for non-classical/silent patients < 45 years old at diagnosis. At 10 years, event-free rates were 25%, 47%, 51% and 60% for these four groups, respectively.
Event-free follow-up rates according to age at diagnosis and clinical pattern of celiac disease at diagnosis (Oslo classification [25]). Group 1: Patients diagnosed at age ≥ 45 years with classical CD. Group 2: Patients diagnosed at age ≥ 45 years with non-classical/silent CD. Group 3: Patients diagnosed at age < 45 years with classical CD. Group 4: Patients diagnosed at age < 45 years with non-classical/silent CD
Discussion
This retrospective study, conducted on data collected over the last two decades in a referral centre, provides a real-world overview on the etiologies, natural history, and clinical predictors of persistent, recurrent or newly developing symptoms both related and unrelated to CD in adult celiac patients on a long-term GFD. Major findings include the identification of celiac patients at risk of having CREs over follow-up, potentially providing new clinical perspectives on follow-up modalities for adult CD.
Firstly, we have shown that functional gastrointestinal disorders were the leading cause (30% of cases) of clinical events during follow-up, whereas etiologies strictly related to CD, which included inadequate adherence to a GFD and malignant complications of CD were rare, accounting, respectively, for only 4% and 2% of all events. Although functional gastrointestinal disorders are not life-threatening conditions, their impact on patients’ quality of life is burdensome [26, 27]. Their association with CD has been known for a long time [18, 28, 29]; however, mechanisms underlying functional symptoms in celiac patients on a long-term GFD are still unclear [12,13,14,15,16,17,18, 30,31,32,33]. Based on our results, the high prevalence of functional gastrointestinal disorders, gastroesophageal reflux disease and micronutrient deficiencies while on a GFD suggest a possible role for dietary quality rebalancing, as crucial intervention, in addition to maintaining a strict lifelong GFD adherence. Recently, it has been reported that gene expression in the small intestine of treated celiac patients differed from healthy controls, particularly for genes involved in the transport of micronutrients [34, 35], therefore this may have implications for nutritional supplementation, especially after diagnosis.
Secondly, one third of patients had at least one clinically relevant event in the first five years after diagnosis. Just under 15% of patients without previous events subsequently developed events between five and ten years from diagnosis. Very few developed events after 10 years. This suggests that strict follow-up immediately after diagnosis is necessary, and then it can be organized on a case-by case basis. We have shown that age (> 45 years old) and classical pattern at diagnosis of CD were independent predictors of CREs. These parameters may be used to stratify patients into three subgroups according to their risk of developing CREs during follow-up, regardless of severity or etiological relationship with CD. These results complete our previous findings that age at diagnosis and clinical pattern of CD were risk factors for the development of malignant complications of CD [11].
Currently, international guidelines recommend regular follow-up in adult CD, but timing and modalities of follow-up are not standardised [1,2,3]. Implications for clinical practice of our results include the development of personalized and cost-effective modalities for the follow-up of adult celiac patients, including timing of follow-up, role of healthcare practitioners, and decentralization of care for those at low risk. We believe a possible cost-effective proposal may be as follows: (1) for patients diagnosed at age ≥ 45 years with classical symptoms maintenance of regular annual follow-up specialist medical consultation; (2) for patients diagnosed at age < 45 years with non-classical/silent presentation annual specialist medical consultation for the first two years since diagnosis and subsequent discharge to general practice or dietitians with referrals as necessary; (3) annual follow-up for the first five years and then on a case-by-case basis in the remaining patients. These last two scenarios account for the vast majority of celiac patients in our cohort.
This study has some limitations, which are predominantly related to the retrospective and single-centre design in a referral center, limited sample size and lack of standardized methods to retrospectively assess and categorize the heterogeneous symptoms, which develop in celiac patients. The referral centre setting may limit generalizability of our results, given the lack of data on patient outcomes in other settings such as general practice and other secondary care settings. However, it is extremely difficult to obtain data on celiac patients followed-up in these settings.
We did not find adherence to a GFD or persistence of VA on follow-up duodenal biopsy as predictors of CREs. In our cohort, only a minority of patients had clinical events attributable to poor adherence to a GFD. This is in contrast with the current literature, which reports voluntary/involuntary dietary lapses as the most common cause of NRCD (persistent/recurrent symptoms and/or villous atrophy) [12,13,14,15,16,17,18]. Reasons behind this may include discrepancies in methods for assessing GFD adherence, cultural differences due to the culinary and social background and strict instruction received by our patients on how to maintain lifelong rigorous dietary adherence [23, 24].
It should be noted that although persistence of VA at follow-up duodenal biopsy did not reach significance on multivariate analysis, this may be due to our limited sample size. Nonetheless, the literature provides discordant data on the relationship between persistent VA during follow-up and risk of poor long-term outcomes in celiac patients [16, 36, 37].
In conclusion, this study has delineated the natural history, etiologies and predictors of CREs in patients with CD on a long-term GFD and provided a proposal for a cost-effective optimization of follow-up care of these patients. Although requiring a confirmation on larger sample sizes, and integration with emerging methods for implementing adherence to a GFD such as gluten peptides [38], we hope these results can help clinicians to deliver the best quality of care to celiac patients. Finally, our results may potentially be relevant in selecting patients for no-biopsy diagnostic strategies for adult CD, considering the growing interest in this approach [5, 6].
Change history
27 May 2023
The co-author "Federico Biagi" e-mail address has been corrected.
References
Ludvigsson JF, Bai JC, Biagi F et al. Diagnosis and management of adult coeliac disease: guidelines from the British Society of Gastroenterology. Gut 2014;63:1210–1228.
Rubio-Tapia A, Hill ID, Kelly CP et al. ACG clinical guidelines: diagnosis and management of celiac disease. Am. J. Gastroenterol. 2013;108:656–676.
Al-Toma A, Volta U, Auricchio R et al. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United Eur. Gastroenterol. J. 2019;7:583–613.
Singh P, Arora A, Strand TA, Leffler DA, Catassi C, Green PH, Kelly CP, Ahuja V, Makharia GK. Global prevalence of celiac disease: systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2018;16(6):823–836.
Fuchs V, Kurppa K, Huhtala H et al. Serology-based criteria for adult coeliac disease have excellent accuracy across the range of pre-test probabilities. Aliment. Pharmacol. Ther. 2019;49:277–284.
Penny HA, Raju SA, Lau MS et al. Accuracy of a no-biopsy approach for the diagnosis of coeliac disease across different adult cohorts. Gut 2021;70:876–883.
Holmes GK, Prior P, Lane MR et al. Malignancy in celiac disease–effect of a gluten free diet. Gut 1989;30:333–338.
Hall NJ, Rubin G, Charnock A. Systematic review: adherence to a gluten-free diet in adult patients with celiac disease. Aliment. Pharmacol. Ther. 2009;30:315–330.
Zanchetta MB, Longobardi V, Bai JC. Bone and celiac disease. Curr. Osteoporos. Rep. 2016;14:43–48.
Schiepatti A, Sprio E, Sanders DS et al. Coeliac disease and obstetric and gynaecological disorders: where are we now? Eur. J. Gastroenterol. Hepatol. 2019;31:425–433.
Biagi F, Schiepatti A, Maiorano G et al. Risk of complications in celiac patients depends on age at diagnosis and type of clinical presentation. Dig. Liver Dis. 2018;50:549–552.
Penny HA, Baggus EMR, Rej A et al. Non-responsive coeliac disease: a comprehensive review from the NHS England National Centre for Refractory Coeliac Disease. Nutrients 2020;12:216.
Leffler DA, Dennis M, Hyett B et al. Etiologies and predictors of diagnosis in nonresponsive celiac disease. Clin. Gastroenterol. Hepatol. 2007;5:445–450.
O’Mahony S, Howdle PD, Losowsky MS. Review article: management of patients with non-responsive coeliac disease. Aliment. Pharmacol. Ther. 1996;10:671–680.
Abdulkarim AS, Burgart LJ, See J, Murray JA. Etiology of nonresponsive celiac disease: results of a systematic approach. Am. J. Gastroenterol. 2002;97:2016–2021.
Kaukinen K, Peräaho M, Lindfors K et al. Persistent small bowel mucosal villous atrophy without symptoms in coeliac disease. Aliment. Pharmacol. Ther. 2007;15:1237–1245.
Stasi E, Marafini I, Caruso R et al. Frequency and cause of persistent symptoms in celiac disease patients on a long-term gluten-free diet. J. Clin. Gastroenterol. 2016;50:239–243.
Fine KD, Meyer RL, Lee EL. The prevalence and causes of chronic diarrhea in patients with celiac sprue treated with a gluten-free diet. Gastroenterology 1997;112:1830–1838.
Al-Toma A, Verbeek WH, Hadithi M et al. Survival in refractory coeliac disease and enteropathy-associated T-cell lymphoma: retrospective evaluation of single-centre experience. Gut 2007;56:1373–1378.
Rubio-Tapia A, Kelly DG, Lahr BD et al. Clinical staging and survival in refractory celiac disease: a single center experience. Gastroenterology 2009;136:99–353.
Biagi F, Marchese A, Ferretti F et al. A multicentre case control study on complicated coeliac disease: two different patterns of natural history, two different prognoses. BMC Gastroenterol. 2014;14:139.
Biagi F, Vattiato C, Agazzi S et al. A second duodenal biopsy is necessary in the follow-up of adult coeliac patients. Ann. Med. 2014;46:430–433.
Biagi F, Andrealli A, Bianchi PI et al. A gluten-free diet score to evaluate dietary compliance in patients with coeliac disease. Br. J. Nutr. 2009;102:882–887.
Schiepatti A, Maimaris S, Nicolardi ML et al. Determinants and trends of adherence to a gluten-free diet in adult celiac patients on a long-term follow-up (2000–2020). Clin. Gastroenterol. Hepatol. 2022;20:e741–e749.
Ludvigsson JF, Leffler DA, Bai JC et al. The Oslo definitions for coeliac disease and related terms. Gut 2013;62:43–52.
Parker S, Palsson O, Sanders DS et al. functional gastrointestinal disorders and associated health impairment in individuals with celiac disease. Clin. Gastroenterol. Hepatol. 2021;S1542–3565:00758–00768.
Zingone F, Swift GL, Card TR et al. Psychological morbidity of celiac disease: a review of the literature. United Eur. Gastroenterol. J. 2015;3:136–145.
Sanders DS, Carter MJ, Hurlstone DP et al. Association of adult coeliac disease with irritable bowel syndrome: a case-control study in patients fulfilling ROME II criteria referred to secondary care. Lancet 2001;358:1504–1508.
Sainsbury A, Sanders DS, Ford AC. Prevalence of irritable bowel syndrome-type symptoms in patients with celiac disease: a meta-analysis. Clin. Gastroenterol. Hepatol. 2013;11:359–365.
Ludvigsson JF, Lebwohl B, Chen Q et al. Anxiety after coeliac disease diagnosis predicts mucosal healing: a population-based study. Aliment. Pharmacol. Ther. 2018;48:1091–1098.
Schiepatti A, Bellani V, Perlato M et al. Inadvertent and minimal gluten intake has a negligible role in the onset of symptoms in patients with coeliac disease on a gluten-free diet. Br. J. Nutr. 2019;121:576–581.
Wacklin P, Laurikka P, Lindfors K et al. Altered duodenal microbiota composition in celiac disease patients suffering from persistent symptoms on a long-term gluten-free diet. Am. J. Gastroenterol. 2014;109:1933–1941.
Schiepatti A, Bacchi S, Biagi F et al. Relationship between duodenal microbiota composition, clinical features at diagnosis, and persistent symptoms in adult coeliac disease. Dig. Liver Dis. 2021;53:972–979.
Dotsenko V, Oittinen M, Taavela J et al. Genome-wide transcriptomic analysis of intestinal mucosa in celiac disease patients on a gluten-free diet and postgluten challenge. Cell. Mol. Gastroenterol. Hepatol. 2021;11:13–32.
Roncoroni L, Bascuñán KA, Doneda L et al. A low FODMAP gluten-free diet improves functional gastrointestinal disorders and overall mental health of celiac disease patients: a randomized controlled trial. Nutrients 2018;10:1023.
Lebwohl B, Granath F, Ekbom A et al. Mucosal healing and mortality in coeliac disease. Aliment. Pharmacol. Ther. 2013;37:332–339.
Rubio-Tapia A, Rahim MW, See JA et al. Mucosal recovery and mortality in adults with celiac disease after treatment with a gluten-free diet. Am. J. Gastroenterol. 2010;105:1412–1420.
Stefanolo JP, Tálamo M, Dodds S et al. Real-world gluten exposure in patients with celiac disease on gluten-free diets, determined from gliadin immunogenic peptides in urine and fecal samples. Clin. Gastroenterol. Hepatol. 2021;19:484–491.
Funding
Open access funding provided by Università degli Studi di Pavia within the CRUI-CARE Agreement. This study received no funding.
Author information
Authors and Affiliations
Contributions
AS and FB planned the study. AS, SM, FL, DS, PM, MC, EF collected the data. SM performed the statistical analysis. AS, SM, FB interpreted the data and drafted the manuscript. All the Authors revised and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the ethical review board of IRCCS Pavia, ICS Maugeri, Pavia, Italy (protocol number 2381 CE, approved on 14th January 2020). The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008) as reflected in a priori approval by the institution's human research committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Schiepatti, A., Maimaris, S., Lusetti, F. et al. High Prevalence of Functional Gastrointestinal Disorders in Celiac Patients with Persistent Symptoms on a Gluten-Free Diet: A 20-Year Follow-Up Study. Dig Dis Sci 68, 3374–3382 (2023). https://doi.org/10.1007/s10620-022-07727-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-022-07727-x