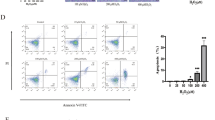
Abstract
Background/Aims
Mesenchymal stem cells (MSCs) are a type of adult pluripotent stem cell that has anti-inflammatory and immunomodulatory effects, and whose conditioned medium (CM) has also been found to be effective. We used MSC and CM enemas to investigate their ameliorative effects in a mouse model of colitis.
Methods
We employed MSCs, CM, and MSCs + ML385 (an inhibitor of Nrf2) in dextran sodium sulfate (DSS)-induced colitis. Mice were sacrificed on day 8, and the effects of MSC or CM treatment on the levels of inflammation and oxidative stress in colonic epithelial cells were evaluated by histological analyses.
Results
MSCs inhibited inflammatory cell infiltration and proinflammatory cytokine expression in the colon. In addition, MSCs reduced extracellular matrix deposition and maintained the mechanical barrier and permeability of colonic epithelial cells. Mechanistically, MSCs activated Nrf2, which then increased HO-1 and NQO-1 levels and downregulated the expression of Keap1 to suppress reactive oxygen species production and MDA generation, accompanied by increases in components of the enzymatic antioxidant system, including SOD, CAT, GSH-Px, and T-AOC. However, after administering an Nrf2 inhibitor (ML385) to block the Nrf2/Keap1/ARE pathway, we failed to observe protective effects of MSCs in mice with colitis. CM alone also produced some of the therapeutic benefits of MSCs but was not as effective as MSCs.
Conclusions
Our data confirmed that MSCs and CM can effectively improve intestinal mucosal repair in experimental colitis and that MSCs can improve this condition by activating the Nrf2/Keap1/ARE pathway.





Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
References
Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nature Reviews Gastroenterology & Hepatology 2015;12:205–217.
Studd C, Cameron G, Beswick L et al. Never underestimate inflammatory bowel disease: high prevalence rates and confirmation of high incidence rates in Australia. Journal of Gastroenterology and Hepatology 2016;31:81–86.
Jiang Y, Jahagirdar BN, Reinhardt RL et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002;418:41–49.
Ankrum J, Karp JM. Mesenchymal stem cell therapy: two steps forward, one step back. Trends in Molecular Medicine 2010;16:203–209.
Shi Y, Su J, Roberts AI et al. How mesenchymal stem cells interact with tissue immune responses. Trends in Immunology 2012;33:136–143.
Han Y, Li X, Zhang Y et al. Mesenchymal stem cells for regenerative medicine. Cells 2019;8:886.
Burdon TJ, Paul A, Noiseux N et al. Bone marrow stem cell derived paracrine factors for regenerative medicine: current perspectives and therapeutic potential. Bone Marrow Research 2011;2011:207326.
García-Olmo D, García-Arranz M, Herreros D et al. A phase I clinical trial of the treatment of Crohn’s fistula by adipose mesenchymal stem cell transplantation. Diseases of the Colon and Rectum 2005;48:1416–1423.
García-Olmo D, Herreros D, De-La-Quintana P et al. Adipose-derived stem cells in Crohn’s rectovaginal fistula. Case Reports in Medicine 2010;2010:961758.
Garcia-Olmo D, Herreros D, Pascual I et al. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Diseases of the Colon and Rectum 2009;52:79–86.
Garcia-Olmo D, Herreros D, Pascual M et al. Treatment of enterocutaneous fistula in Crohn’s disease with adipose-derived stem cells: a comparison of protocols with and without cell expansion. International Journal of Colorectal Disease 2009;24:27–30.
Shi X, Chen Q, Wang F. Mesenchymal stem cells for the treatment of ulcerative colitis: a systematic review and meta-analysis of experimental and clinical studies. Stem Cell Research & Therapy 2019;10:266.
Dietz A, Dozois E, Fletcher J et al. Autologous mesenchymal stem cells, applied in a bioabsorbable matrix, for treatment of perianal fistulas in patients with Crohn’s disease. Gastroenterology 2017;153:59-62.e52.
Sala E, Genua M, Petti L et al. Mesenchymal stem cells reduce colitis in mice via release of TSG6, independently of their localization to the intestine. Gastroenterology 2015;149:163-176.e120.
Kim H, Shin T, Lee B et al. Human umbilical cord blood mesenchymal stem cells reduce colitis in mice by activating NOD2 signaling to COX2. Gastroenterology 2013;145:1392-1403.e1391–1398.
Li Q, Xu Y, Lv K et al. Small extracellular vesicles containing miR-486–5p promote angiogenesis after myocardial infarction in mice and nonhuman primates. Science Translational Medicine. 2021. https://doi.org/10.1126/scitranslmed.abb0202.
Dorronsoro A, Santiago F, Grassi D et al. Mesenchymal stem cell-derived extracellular vesicles reduce senescence and extend health span in mouse models of aging. Aging Cell 2021;20:e13337.
Kuo SC, Chio CC, Yeh CH et al. Mesenchymal stem cell-conditioned medium attenuates the retinal pathology in amyloid-β-induced rat model of Alzheimer’s disease: underlying mechanisms. Aging Cell. 2021;20:e13340. https://doi.org/10.1111/acel.13340.
Hickson L, Eirin A, Conley S et al. Diabetic kidney disease alters the transcriptome and function of human adipose-derived mesenchymal stromal cells but maintains immunomodulatory and paracrine activities important for renal repair. Diabetes 2021;70:1561–1574.
Willis G, Reis M, Gheinani A et al. Extracellular vesicles protect the neonatal lung from hyperoxic injury through the epigenetic and transcriptomic reprogramming of myeloid cells. American Journal of Respiratory and Critical Care Medicine. 2021. https://doi.org/10.1164/rccm.202102-0329OC.
Huang J, Kin Pong U, Yang F et al. Human pluripotent stem cell-derived ectomesenchymal stromal cells promote more robust functional recovery than umbilical cord-derived mesenchymal stromal cells after hypoxic–ischaemic brain damage. Theranostics 2022;12:143–166.
Ni S, Wang D, Qiu X et al. Bone marrow mesenchymal stem cells protect against bleomycin-induced pulmonary fibrosis in rat by activating Nrf2 signaling. International Journal of Clinical and Experimental Pathology 2015;8:7752–7761.
Yang L, Shen ZY, Wang RR et al. Effects of heme oxygenase-1-modified bone marrow mesenchymal stem cells on microcirculation and energy metabolism following liver transplantation. World Journal of Gastroenterology 2017;23:3449–3467.
Wang M, Liang C, Hu H et al. Intraperitoneal injection (IP), Intravenous injection (IV) or anal injection (AI)? Best way for mesenchymal stem cells transplantation for colitis. Scientific Reports 2016;6:30696.
Hayashi Y, Tsuji S, Tsujii M et al. Topical implantation of mesenchymal stem cells has beneficial effects on healing of experimental colitis in rats. The Journal of Pharmacology and Experimental Therapeutics 2008;326:523–531.
Robinson AM, Miller S, Payne N et al. Neuroprotective potential of mesenchymal stem cell-based therapy in acute stages of TNBS-induced colitis in guinea-pigs. PLoS ONE 2015;10:e0139023.
Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell 2009;4:206–216.
Bier A, Berenstein P, Kronfeld N et al. Placenta-derived mesenchymal stromal cells and their exosomes exert therapeutic effects in Duchenne muscular dystrophy. Biomaterials 2018;174:67–78.
Jiang W, Tan Y, Cai M et al. Human umbilical cord MSC-derived exosomes suppress the development of CCl(4)-induced liver injury through antioxidant effect. Stem Cells International 2018;2018:6079642.
Cunningham CJ, Redondo-Castro E, Allan SM. The therapeutic potential of the mesenchymal stem cell secretome in ischaemic stroke. Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism 2018;38:1276–1292.
Harrell CR, Simovic Markovic B, Fellabaum C et al. Therapeutic potential of mesenchymal stem cell-derived exosomes in the treatment of eye diseases. Advances in Experimental Medicine and Biology 2018;1089:47–57.
Wang T, Jian Z, Baskys A et al. MSC-derived exosomes protect against oxidative stress-induced skin injury via adaptive regulation of the NRF2 defense system. Biomaterials 2020;257:120264.
Turner D, Ricciuto A, Lewis A et al. STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology 2021;160:1570–1583.
Acknowledgments
We thank Dr. Canxia Xu at The Third Xiangya Hospital of Central South University for the critical comments to the manuscript.
Funding
This work received support from Independent Exploration and Innovation project for postgraduate of Central South University (grant no. 2021zzts0406), National Natural Science Foundation of China (grant no. 81570509), and Changsha Municipal Natural Science Foundation (grant no. kq2014257).
Author information
Authors and Affiliations
Contributions
L.P., X.X.R., W.H., C.J.S., and L.H. conceived and designed the study, conducted the experiments, interpreted the data, and prepared the manuscript. L.X.M., T.Y., and L.J. take responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10620_2022_7722_MOESM1_ESM.jpg
Supplementary file1 (JPG 1117 KB) Supplementary Fig. 1. Distribution of GFP+ MSCs in colon 2 day after treatment. A GFP was transfected to MSC, and the GFP-labeling was confirmed. B GFP + cells were detected at the inflamed colon. On the fourth day of the mouse model of colitis, both healthy mice and colitis mice were anal injected with GFP + MSCs (1 × 106, 200 μL in volume). In the control group, no cells clustered in the intestinal mucosa; In the DSS-modeling group, cells were found accumulation at the lumen. Magnification: × 40. Scale bar, 100 μm
10620_2022_7722_MOESM2_ESM.jpg
Supplementary file1 (JPG 2933 KB) Supplementary Fig. 2. Therapeutic effects of untreated culture medium (UCM) in DSS-induced colitis mice. A Representative colon images and quantification of colon length. B Distal colons were removed and sectioned followed by H&E staining. Representative sections are displayed. Inflammation scores were evaluated in bar graph. C IHC analysis for the macrophage specific marker CD68 was performed and subsequently positive cells were evaluated. D IHC analysis for the neutrophil specific marker MPO was performed and subsequently positive cells were evaluated. The insets are magnified images of the indicated rectangles. Magnification: × 100. Scale bar, 200 μm. Values are the means ± SD. n = 6 animals per group per time point. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001; ns no significance
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, P., Xie, Xr., Wu, H. et al. Mesenchymal Stem Cells Promote Intestinal Mucosal Repair by Positively Regulating the Nrf2/Keap1/ARE Signaling Pathway in Acute Experimental Colitis. Dig Dis Sci 68, 1835–1846 (2023). https://doi.org/10.1007/s10620-022-07722-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-022-07722-2