Abstract
Background
The laminin gamma 1 chain (LMγ1) is abundant along the crypt-villus axis in the intestinal basement membrane.
Aims
We investigated whether a serological biomarker of laminin degradation was associated with disease activity in patients with Crohn’s disease (CD) and in rats with dextran sulfate sodium (DSS)-induced colitis.
Methods
Serum samples from CD patients (n = 43), healthy subjects (n = 19), and Sprague Dawley rats receiving 5–6% DSS water for five days and regular drinking water for 11 days were included in this study. The LG1M biomarker, a neo-epitope degradation fragment of the LMγ1 chain generated by matrix metalloproteinases-9 (MMP-9), was measured in serum to estimate the level of laminin degradation.
Results
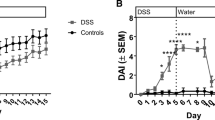
Serum LG1M was elevated in CD patients with active and inactive disease compared to healthy subjects (p < 0.0001). LG1M distinguished CD patients from healthy subjects, with an area under the curve (AUC) of 0.81 (p < 0.0001). Serum LG1M was decreased in DSS rats compared to controls 2 days after DSS withdrawal, and increased upon reversal of the disease.
Conclusions
Increased serum LG1M in active and inactive CD patients supports the evidence of altered LM expression in both inflamed and non-inflamed tissue. Moreover, lower LG1M levels in the early healing phase of DSS-induced colitis may reflect ongoing mucosal repair.




Similar content being viewed by others
References
Giuffrida P, Corazza GR, Di Sabatino A. Old and new lymphocyte players in inflammatory bowel disease. Dig Dis Sci 2018;63:277–288. https://doi.org/10.1007/s10620-017-4892-4.
Vermeire S, Van Assche G, Rutgeerts P. C-reactive protein as a marker for inflammatory bowel disease. Inflamm Bowel Dis 2004;10:661–665. https://doi.org/10.1097/00054725-200409000-00026.
Chang S, Malter L, Hudesman D. Disease monitoring in inflammatory bowel disease. World J Gastroenterol 2015;21:11246–11259.
Mortensen JH, Godskesen LE, Jensen MD et al. Fragments of citrullinated and MMP-degraded vimentin and MMP-degraded type III collagen are novel serological biomarkers to differentiate Crohn’s disease from ulcerative colitis. J Crohns Colitis 2015;9:863–872.
Mortensen JH, Manon-Jensen T, Jensen MD et al. Ulcerative colitis, Crohn’s disease, and irritable bowel syndrome have different profiles of extracellular matrix turnover, which also reflects disease activity in Crohn’s disease. PLoS One 2017;12:e0185855. https://doi.org/10.1371/journal.pone.0185855.
Van Haaften WT, Mortensen JH, Karsdal MA et al. Misbalance in type III collagen formation/degradation as a novel serological biomarker for penetrating (Montreal B3) Crohn’s disease. Aliment Pharmacol Ther 2017;46:26–39.
Goffin L, Fagagnini S, Vicari A, et al. Anti-MMP-9 antibody: a promising therapeutic strategy for treatment of inflammatory bowel disease complications with fibrosis. Inflamm Bowel Dis 2016;22:2041–2057. http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00054725-201609000-00001.
Giuffrida P, Biancheri P, Macdonald TT. Proteases and small intestinal barrier function in health and disease. Curr Opin Gastroenterol 2014;30:147–153.
Giuffrida P, Pinzani M, Corazza GR, et al. Biomarkers of intestinal fibrosis—one step towards clinical trials for stricturing inflammatory bowel disease. 2016.
Lenti MV, Di Sabatino A. Intestinal fibrosis. Mol Aspects Med 2019;65:100–109. https://doi.org/10.1016/j.mam.2018.10.003.
Simon-Assmann P, Spenle C, Lefebvre O et al. The role of the basement membrane as a modulator of intestinal epithelial-mesenchymal interactions. Amsterdam: Elsevier; 2010. https://doi.org/10.1016/B978-0-12-381280-3.00008-7.
Teller IC, Beaulieu JF. Interactions between laminin and epithelial cells in intestinal health and disease. Expert Rev Mol Med 2001;3:1–18.
Barrett JC, Lee JC, Lees CW, et al. Genome-wide association study of ulcerative colitis identifies three new susceptibility loci, including the HNF4A region. Nat Genet 2009;41:1330–1334. http://www.ncbi.nlm.nih.gov/pubmed/19915572.
Bouatrouss Y, Herring-gillam FE, Gosselin J et al. Altered expression of laminins in Crohn’s disease small intestinal mucosa. Am J Pathol 2000;156:45–50.
Senyürek I, Klein G, Kalbacher H et al. Peptides derived from the human laminin alpha4 and aplha5 chains exhibit antimicrobial activity. Peptides 2010;31:1468–1472.
Yurchenco PD. Integrating activities of laminins that drive basement membrane assembly and function. Amsterdam: Elsevier; 2015. https://doi.org/10.1016/bs.ctm.2015.05.001.
Spenlé C, Hussenet T, Lacroute J et al. Dysregulation of laminins in intestinal inflammation. Pathol Biol 2012;60:41–47.
Nielsen SH, Rasmussen DGK, Brix S et al. A novel biomarker of laminin turnover is associated with disease progression and mortality in chronic kidney disease. PLoS One 2018;13:1–13.
Shon W-J, Lee Y-K, Shin JH et al. Severity of DSS-induced colitis is reduced in Ido1-deficient mice with down-regulation of TLR-MyD88-NF-kB transcriptional networks. Sci Rep. 2015. https://doi.org/10.1038/srep17305.
Lindholm M, Manon-Jensen T, Madsen GI et al. Extracellular matrix fragments of the basement membrane and the interstitial matrix are serological markers of intestinal tissue remodeling and disease activity in dextran sulfate sodium colitis. Dig Dis Sci 2019;64:3134–3142.
Gaudio E, Taddei G, Vetuschi A et al. Dextran sulfate sodium (DSS) colitis in rats: clinical, structural, and ultrastructural aspects. Dig Dis Sci 1999;44:1458–1475.
Francoeur C, Escaffit F, Vachon PH et al. Proinflammatory cytokines TNF-a and IFN-g alter laminin expression under an apoptosis-independent mechanism in human intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 2004;287:G592–G598.
Koutroubakis IE, Petinaki E, Dimoulios P et al. Serum laminin and collagen IV in inflammato ry bowel disease. J Clin Pathol 2003;56:817–821.
Koshikawa N, Giannelli G, Cirulli V et al. Role of cell surface metalloprotease MT1-MMP in epithelial cell migration over laminin 5. J Cell Biol 2000;148:615–624.
Schmehl K, Florian S, Jacobasch G et al. Deficiency of epithelial basement membrane laminin in ulcerative colitis affected human colonic mucosa. Int J Colorectal Dis 2000;15:39–48.
Turck N, Gross I, Gendry P et al. Laminin isoforms: biological roles and effects on the intracellular distribution of nuclear proteins in intestinal epithelial cells. Exp Cell Res 2005;303:494–503.
Simon-Assmann P, Kedinger M, De Arcangelis A et al. Extracellular matrix components in intestinal development. Experientia 1995;51:883–900.
Trier JS, Allan CH, Abrahamson DR et al. Epithelial basement membrane of mouse jejunum. Evidence for laminin turnover along the entire crypt-villus axis. J Clin Invest 1990;86:87–95.
Mikami H, Watanabe S, Hirose M, et al. Role of extracellular matrix in wound repair by cultured gastric mucosal cells. Biochem Biophys Res Commun 1994;202:285–292. http://www.ncbi.nlm.nih.gov/pubmed/8037723.
Hussey GS, Keane TJ, Badylak SF. The extracellular matrix of the gastrointestinal tract: a regenerative medicine platform. Nat Rev Gastroenterol Hepatol. 2017. https://doi.org/10.1038/nrgastro.2017.76.
Funding
This work was supported by the Danish Research Foundation and the Innovation Fund Denmark.
Author information
Authors and Affiliations
Contributions
ML designed the animal study, collected data, performed data analysis, interpreted data, drafted the manuscript, and revised critically for important intellectual content. ADS, GM, PG, and MP designed the clinical study, collected data, and revised critically for important intellectual content. GIM scored the histological specimens and revised critically for important intellectual content. TMJ, AK, MAK, JK, and JHM designed the study, interpreted data, and revised critically for important intellectual content.
Corresponding author
Ethics declarations
Conflict of interest
ML, TMJ, MAK, and JHM are full-time employees at Nordic Bioscience. TMJ and MAK hold stock in Nordic Bioscience. Nordic Bioscience is a privately owned small-medium-sized enterprise, partly focused in the development of biomarkers for connective tissue disorders and rheumatic diseases. None of the authors received any kind of financial benefits or other bonuses for the work described in this manuscript. ADS, GM, GIM, PG, MP, AK, and JK have no financial disclosures or conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Summary: This manuscript reports an altered remodeling of the basement membrane protein laminin γ1 chain in CD patients and in DSS rats. This is done with the use of a serological biomarker, LG1M, a MMP-9 generated neo-epitope degradation fragment of the LMγ1 chain.
Rights and permissions
About this article
Cite this article
Lindholm, M., Di Sabatino, A., Manon-Jensen, T. et al. A Serological Biomarker of Laminin Gamma 1 Chain Degradation Reflects Altered Basement Membrane Remodeling in Crohn’s Disease and DSS Colitis. Dig Dis Sci 67, 3662–3671 (2022). https://doi.org/10.1007/s10620-021-07252-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-021-07252-3