Abstract
Background
Clinical observations indicate that mechanical factors contribute to the expression or recurrence of Crohn’s disease. We investigated whether the creation of an intestinal stenosis could alter the severity of the expected Crohn-like ileitis, in a Crohn’s disease animal model, the TNFΔare/+ mouse.
Methods
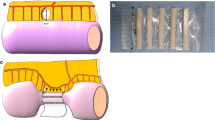
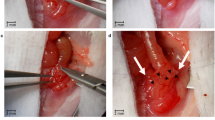
Thirty-six, 6-weeks-old TNFΔare/+ mice, were divided into 3 intervention groups: triple suture, single suture and sham. In the terminal ileum, in the first group, a triple suture stenosis was created, whereas, in the second, a loose suture was placed. Same triple-suture stenosis was performed on twelve wild type mice. All animals were sacrificed at 6 weeks post-operatively and the ileum parts were evaluated histopathologically. A summative total ileitis score was applied in each sample using a bespoke semiquantitative histological scoring system for the Crohn-like changes.
Results
The triple suture stenosis induced significant muscular hypertrophy proximal to interventional site which was more prominent in TNFΔare/+ than wild type mice. In triple suture group, the total ileitis score was significantly increased proximal to the intervention as compared to the single suture (P: 0.004) and the sham groups (P: 0.013). The total ileitis score distally, was unaffected, regardless of the experimental intervention. Intestinal stenosis did not induce intestinal inflammation in wild type mice.
Conclusion
The creation of a stenosis in the terminal ileum of TNFΔare/+ mice alters Crohn-like inflammation. We assume that mechanical forces, such as intraluminal pressure, may contribute as important co-factors to the pathophysiology of Crohn’s disease in genetically predisposed subjects.







Similar content being viewed by others
References
Crohn BB, Ginzburg L, Oppenheimer GD. Regional ileitis: a pathologic and clinical entity. J Am Med Assoc. 1932;99:1323–1329.
Gajendran M, Loganathan P, Catinella AP, Hashash JG. A comprehensive review and update on Crohn’s disease. Disease-a-Month. 2018;64:20–57. https://doi.org/10.1016/j.disamonth.2017.07.001.
Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn’s disease. Lancet. 2017;389:1741–1755.
Caprilli R. Why does Crohn ’ s disease usually occur in terminal ileum ? J Crohn’s Colitis 2008;2:352–366. https://doi.org/10.1016/j.crohns.2008.06.001.
Pizarro TT, Arseneau KO, Bamias G, Cominelli F. Mouse models for the study of Crohn’s disease. Trends Mol Med. 2003;9:218–222.
Cominelli F, Arseneau KO, Rodriguez-Palacios A, Pizarro TT. Uncovering pathogenic mechanisms of inflammatory bowel disease using mouse models of Crohn’s disease-like ileitis: what is the right model? Cell Mol Gastrenterol Hepatol 2017;4:19–32. https://doi.org/10.1016/j.jcmgh.2017.02.010.
Mizoguchi A. Animal models of inflammatory bowel disease. In: Progress in molecular biology and translational science. Elsevier B.V.; 2012, pp 263–320.
Kontoyiannis D, Boulougouris G, Manoloukos M, Armaka M, Apostolaki M, Pizarro T et al. Genetic dissection of the cellular pathways and signaling mechanisms in modeled tumor necrosis factor—induced Crohn ’ s-like Inflammatory bowel disease. J Exp Med. 2002;196:1563–1574.
Reynolds HL, Stellato TA. Crohn’s disease of the foregut. Surg Clin North Am. 2020;81:117–135.
Gionchetti P, Dignass A, Danese S, Dias FJM, Rogler G, Lakatos PL et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 2: Surgical management and special situations. J Crohn’s Colitis 2017;11:135–149.
Gklavas A, Dellaportas D, Papaconstantinou I. Risk factors for postoperative recurrence of crohn’s disease with emphasis on surgical predictors. Ann Gastroenterol. 2017;30:598–612.
Cima RR, Wolff BG. Reoperative Crohn ’ s surgery: tricks of the trade. Intest Res 2007;1:336–343.
Roulis M, Armaka M, Manoloukos M, Apostolaki M, Kollias G. Intestinal epithelial cells as producers but not targets of chronic TNF suffice to cause murine Crohn-like pathology. Proc Natl Acad Sci USA 2011;108:5396–5401.
Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514–521.
Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU- rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999;10:387–398.
Baur P, Martin F-P, Gruber L, Bosco N, Brahmbhatt V, Collino S et al. Metabolic phenotyping of the Crohn’s disease-like IBD etiopathology in the TNF ΔARE/WT mouse model. J Proteome Res. 2011;10:5523–5535.
Gibson PR. Increased gut permeability in Crohn’s disease: is TNF the link ? Gut. 2004;53:1724–1729.
The European Parliament and the Council of the European Union. Directive 2010/63/EU of the European Paliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Off J Eur Union. 2010;(276):33–79.
President of the Greek Republic. Presidential Decree 56/2013. Εφημερίδα Της Κυβερνήσεως. 2013;(53).
National Centre for the Refinement & Reduction of Animals in Research. The 3Rs | NC3Rs [Internet]. [cited 2021 Jan 31]. https://www.nc3rs.org.uk/the-3rs
Brito MVH, Yasojima EY, Teixeira RKC, de Houat AP, Yamaki VN, da Costa FLS. Fasting does not induce gastric emptying in rats. Acta Cir Bras 2015;30:165–169.
Ha SE, Wei L, Jorgensen BG, Lee MY, Park PJ, Poudrier SM et al. A mouse model of intestinal partial obstruction. J Vis Exp. 2018;133:1–7.
Berlin SC, Goske MJ, Obuchowski N, Alexander F, Zepp RC, Goldblum JR et al. Small bowel obstruction in rats: diagnostic accuracy of sonography versus radiography. J Ultrasound Med. 1998;17:497–504.
Georgopoulos I, Mavrigiannaki E, Stasinopoulou S, Renieris G, Nikolakis G, Chaniotakis I et al. Experimental models of partial intestinal obstruction in young mice, establishment, and evaluation. J Surg Res 2020;252:206–215. https://doi.org/10.1016/j.jss.2020.03.007.
American Veterinary Medical Association. AVMA guidelines for the Euthanasia of Animals: 2013 Edition [Internet]. 2013. https://www.avma.org/kb/policies/documents/euthanasia.pdf. (January 2018)
National Research Council of the National Academies. Guide for the care and use of laboratory animals [Internet]. 2011. http://www.ncbi.nlm.nih.gov/pubmed/21595115
Katakura K, Lee J, Rachmilewitz D, Li G, Eckmann L, Raz E. Toll-like receptor 9-induced type I IFN protects mice from experimental colitis. J Clin Invest. 2005;115:695–702.
Dutta S, Sengupta P. Men and mice: relating their ages. Life Sci 2016;152:244–288. https://doi.org/10.1016/j.lfs.2015.10.025.
Velazco C, McMachon L, Ostlie D. Inflammatory bowel disease. In: Holcomb GW, Murphy JP, StPeter SD, Gatti JM, eds. 7th edn. Amsterdam: Elsevier; 2019; 647–663.
Holvoet T, Devriese S, Castermans K, Boland S, Leysen D, Vandewynckel Y-P et al. Treatment of intestinal fibrosis in experimental inflammatory bowel disease by the pleiotropic actions of a local rho kinase inhibitor. Gastroenterology 2017;153:1054–1067.
Rieder F, Kessler S, Sans M, Fiocchi C. Animal models of intestinal fibrosis: new tools for the understanding of pathogenesis and therapy of human disease. Am J Physiol Gastrointest Liver Physiol 2012;303:G786–G801.
Chen W, Lu C, Hirota C, Iacucci M, Ghosh S, Gui X. Smooth muscle hyperplasia/hypertrophy is the most prominent histological change in Crohn’s fibrostenosing bowel strictures: a semiquantitative analysis by using a novel histological grading scheme. J Crohn’s Colitis 2017;11:92–104.
Pizarro TT, Pastorelli L, Bamias G, Garg RR, Reuter BK, Mercado JR et al. The SAMP1/YitFc mouse strain: a spontaneous model of Crohn’s disease-like ileitis. Inflamm Bowel Dis 2011;17:2566–2584.
Zhao J, Liao D, Yang J, Gregersen H. Phasic and tonic smooth muscle function of the partially obstructed guinea pig intestine. J Biomed Biotechnol. 2011;2011:1–9.
Gabella G. Hypertrophy of intestinal smooth muscle. Cell Tissue Res. 1975;163:199–214.
Nair DG, Miller KG, Lourenssen SR, Blennerhassett MG. Inflammatory cytokines promote growth of intestinal smooth muscle cells by induced expression of PDGF-Rβ. J Cell Mol Med. 2014;18:444–454.
Nair DG, Han TY, Lourenssen S, Blennerhassett MG. Proliferation modulates intestinal smooth muscle phenotype in vitro and in colitis in vivo. Am J Physiol Gastrointest Liver Physiol. 2011;300:903–913.
Arrieta MC, Bistritz L, Meddings JB. Alterations in intestinal permeability. Gut 2006;55:1512–1520.
Roulis M, Bongers G, Armaka M, Salviano T, He Z, Singh A et al. Host and microbiota interactions are critical for development of murine Crohn’s-like ileitis. Mucosal Immunol. 2016;9:787–797.
Schaubeck M, Clavel T, Calasan J, Lagkouvardos I, Haange SB, Jehmlich N et al. Dysbiotic gut microbiota causes transmissible Crohn’s disease-like ileitis independent of failure in antimicrobial defence. Gut. 2016;65:225–237.
Dinning PG, Bampton PA, Kennedy ML, Kajimoto T, Lubowski DZ, Carle DJDE et al. Basal pressure patterns and reflexive motor responses in the human ileocolonic junction. Am J Physiol 1999;276:G331–G340.
Quigley EMM, Borody TJ, Phillips SF, Wienbeck M, Tucker RL, Haddad A. Motility of the terminal ileum and ileocecal sphincter in healthy humans. Gastroenterology 1984;87:857–866. https://doi.org/10.1016/0016-5085(84)90080-5.
Baker WNW, Mann CV. The rectosigmoid junction zone: another sphincter? In: Alimentary sphincters and their disorders. London: Palgrave Macmillan UK; 1981, pp 201–11. https://doi.org/10.1007/978-1-349-03940-1_9
Hill JR, Kelley ML, Schlegel JF, Code CF. Pressure profile of the rectum and anus of healthy persons. Dis Colon Rectum 1960;3:203–209.
Enochsson L, Nylander G, Öhman U. Effects of intraluminal pressure on regional blood flow in obstructed and unobstructed small intestines in the rat. Am J Surg 1982;144:558–561.
Hörmannsperger G, Schaubeck M, Haller D. Intestinal microbiota in animal models of inflammatory diseases. ILAR J. 2015;56:179–191.
Kastl AJ, Terry NA, Wu GD, Albenberg LG. The structure and function of the human small intestinal microbiota: current understanding and future directions. Cmgh 2009;9:33–45. https://doi.org/10.1016/j.jcmgh.2019.07.006.
Acknowledgments
Τhe authors are grateful to professor George Kollias and his colleagues Vasiliki Koliaraki, Michalis Meletiou and Vasileios Ntafis for sharing their experience, facilities and equipment whenever needed for this study. We also thank the veterinarian Dr Ioannis Chaniotakis for his academic input and technical assistance throughout the study.
Funding
No specific funding has been received for this study.
Author information
Authors and Affiliations
Contributions
IG: corresponding author, study design, project development, experiments execution, data collection and analysis, manuscript writing. EM: experiments support and execution, data collection and analysis, manuscript writing and editing. SS: histopathologic evaluation, data analysis and interpretation, manuscript editing. GR: data analysis and interpretation, manuscript writing and editing, statistical analysis. GN: data analysis and interpretation, manuscript editing. GB: data interpretation, manuscript writing and editing. DT: histopathologic evaluation, data analysis and interpretation, manuscript editing. IP: project supervision, study conception and design, manuscript editing. All authors critically revised the manuscript for intellectual content and approved the submitted final version.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Ethics statement
Animal housing and experiments were performed in the animal facilities of the Laboratory of Experimental Surgery and Surgery Research "N.S. Christeas" of the National and Kapodistrian University of Athens (code of the facility EL 25 BIO 05). The study, during all stages, was in compliance with the Presidential Degree 56/2013 [19] which incorporates the EU Directive 2010/63/EU for animal experiments [18] and the Greek law 2015/2001, on the protection of animals used for scientific purposes. Ethical approval was granted by the Ethical Committee of Areteion Univeristy Hospital of National and Kapodistrian University of Athens (No B-154/04-02-2016) and the Department of Agricultural and Veterinary Policy of the Region of Attica of the Hellenic Democracy (No 599017/04-10-19).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
An editorial commenting on this article is available at https://doi.org/10.1007/s10620-021-07170-4.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Georgopoulos, I., Mavrigiannaki, E., Stasinopoulou, S. et al. Experimental Intestinal Stenosis Alters Crohn’s Disease-Like Intestinal Inflammation in Ileitis-Prone Mice. Dig Dis Sci 67, 1783–1793 (2022). https://doi.org/10.1007/s10620-021-07161-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-021-07161-5