Abstract
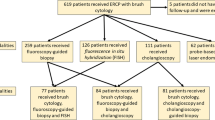
Endoscopic sampling is essential for tissue diagnosis of cholangiocarcinoma (CCA). To evaluate and compare the diagnostic sensitivities of endoscopic retrograde cholangiopancreatography-guided brush cytology biopsy, and endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) in patients with CCA. A comprehensive literature search through multiple databases was conducted for articles published between January 1995 and August 2020. The pooled rates of sensitivity for the diagnosis of CCA and of adverse events were compared among brushing, biopsy, brushing & biopsy, and EUS-FNA. In total, 1123 patients with CCA (32 studies), 719 patients (20 studies), 358 patients (13 studies), and 422 patients (17 studies) were tested by brushing, biopsy, brushing & biopsy, and EUS-FNA, respectively. The pooled diagnostic sensitivity was 56.0% (95% confidence interval (CI) 48.8–63.1%, I2 = 83.0%) with brushing, 67.0% (95% CI 60.2–73.5%, I2 = 72.5%) with biopsy, 70.7% (95% CI 64.1–76.8%, I2 = 42.7%) with brushing & biopsy, and 73.6% (95% CI 64.7–81.5%, I2 = 74.7%) with EUS-FNA. The diagnostic sensitivity was significantly lower for brushing than for biopsy, brushing & biopsy, or EUS-FNA. No significant difference was noted in diagnostic sensitivities among biopsy, brushing & biopsy, and EUS-FNA. Adverse events were comparable between the groups. Intraductal biopsy, brushing & biopsy, and EUS-FNA had comparable efficacy and safety for the diagnosis of CCA. Brushing was the least sensitive diagnostic tool compared with intraductal biopsy or EUS-FNA. Given the modest diagnostic sensitivities of intraductal biopsy and EUS-FNA in the diagnosis of CCA, further studies for complementing these techniques with biomarkers may be needed.


Similar content being viewed by others
References
Banales JM, Marin JJG, Lamarca A et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557–588.
Khan SA, Davidson BR, Goldin RD et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut. 2012;61:1657–1669.
Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145:1215–1229.
Banales JM, Cardinale V, Carpino G et al. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. 2016;13:261–280.
Blechacz B, Komuta M, Roskams T, Gores GJ. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:512–522.
Brandi G, Venturi M, Pantaleo MA, Ercolani G, GICO. Cholangiocarcinoma: Current opinion on clinical practice diagnostic and therapeutic algorithms: A review of the literature and a long-standing experience of a referral center. Dig Liver Dis 2016;48:231–241.
Tsukada K, Takada T, Miyazaki M et al. Diagnosis of biliary tract and ampullary carcinomas. J Hepatobiliary Pancreat Surg. 2008;15:31–40.
Bowlus CL, Olson KA, Gershwin ME. Evaluation of indeterminate biliary strictures. Nat Rev Gastroenterol Hepatol. 2016;13:28–37.
Tummala P, Munigala S, Eloubeidi MA, Agarwal B. Patients with obstructive jaundice and biliary stricture +/- mass lesion on imaging: prevalence of malignancy and potential role of EUS-FNA. J Clin Gastroenterol. 2013;47:532–537.
Roos E, Hubers LM, Coelen RJS et al. IgG4-associated cholangitis in patients resected for presumed perihilar cholangiocarcinoma: a 30-year tertiary care experience. Am J Gastroenterol. 2018;113:765–772.
Navaneethan U, Njei B, Lourdusamy V, Konjeti R, Vargo JJ, Parsi MA. Comparative effectiveness of biliary brush cytology and intraductal biopsy for detection of malignant biliary strictures: a systematic review and meta-analysis. Gastrointest Endosc. 2015;81:168–176.
Forner A, Vidili G, Rengo M, Bujanda L, Ponz-Sarvise M, Lamarca A. Clinical presentation, diagnosis and staging of cholangiocarcinoma. Liver Int. 2019;39:98–107.
Korc P, Sherman S. ERCP tissue sampling. Gastrointest Endosc. 2016;84:557–571.
American Society for Gastrointestinal Endoscopy Standards of Practice C, Anderson MA, Appalaneni V et al. The role of endoscopy in the evaluation and treatment of patients with biliary neoplasia. Gastrointest Endosc. 2013;77:167–174.
Rosch T, Hofrichter K, Frimberger E et al. ERCP or EUS for tissue diagnosis of biliary strictures? A prospective comparative study. Gastrointest Endosc. 2004;60:390–396.
Tellez-Avila FI, Bernal-Mendez AR, Guerrero-Vazquez CG, Martinez-Lozano JA, Ramirez-Luna MA. Diagnostic yield of EUS-guided tissue acquisition as a first-line approach in patients with suspected hilar cholangiocarcinoma. Am J Gastroenterol. 2014;109:1294–1296.
Sadeghi A, Mohamadnejad M, Islami F et al. Diagnostic yield of EUS-guided FNA for malignant biliary stricture: a systematic review and meta-analysis. Gastrointest Endosc. 2016;83:290-298 e291.
De Moura DTH, Moura EGH, Bernardo WM et al. Endoscopic retrograde cholangiopancreatography versus endoscopic ultrasound for tissue diagnosis of malignant biliary stricture: systematic review and meta-analysis. Endosc Ultrasound. 2018;7:10–19.
de Moura DTH, Ryou M, de Moura EGH, Ribeiro IB, Bernardo WM, Thompson CC. Endoscopic ultrasound-guided fine needle aspiration and endoscopic retrograde cholangiopancreatography-based tissue sampling in suspected malignant biliary strictures: a meta-analysis of same-session procedures. Clin Endosc. 2020;53:417–428.
Lee ES, Lee JM. Imaging diagnosis of pancreatic cancer: a state-of-the-art review. World J Gastroenterol. 2014;20:7864–7877.
Singh A, Siddiqui UD. The role of endoscopy in the diagnosis and management of cholangiocarcinoma. J Clin Gastroenterol. 2015;49:725–737.
Rhee H, Park MS. The role of imaging in current treatment strategies for pancreatic adenocarcinoma. Korean J Radiol. 2021;22:23–40.
Shamseer L, Moher D, Clarke M et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647.
Stroup DF, Berlin JA, Morton SC et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605.
Thompson SG, Pocock SJ. Can meta-analyses be trusted? Lancet. 1991;338:1127–1130.
Ponchon T, Gagnon P, Berger F et al. Value of endobiliary brush cytology and biopsies for the diagnosis of malignant bile duct stenosis: results of a prospective study. Gastrointest Endosc. 1995;42:565–572.
Pugliese V, Conio M, Nicolo G, Saccomanno S, Gatteschi B. Endoscopic retrograde forceps biopsy and brush cytology of biliary strictures: a prospective study. Gastrointest Endosc. 1995;42:520–526.
Sugiyama M, Atomi Y, Wada N, Kuroda A, Muto T. Endoscopic transpapillary bile duct biopsy without sphincterotomy for diagnosing biliary strictures: a prospective comparative study with bile and brush cytology. Am J Gastroenterol. 1996;91:465–467.
Mansfield JC, Griffin SM, Wadehra V, Matthewson K. A prospective evaluation of cytology from biliary strictures. Gut. 1997;40:671–677.
Schoefl R, Haefner M, Wrba F et al. Forceps biopsy and brush cytology during endoscopic retrograde cholangiopancreatography for the diagnosis of biliary stenoses. Scand J Gastroenterol. 1997;32:363–368.
Glasbrenner B, Ardan M, Boeck W, Preclik G, Moller P, Adler G. Prospective evaluation of brush cytology of biliary strictures during endoscopic retrograde cholangiopancreatography. Endoscopy. 1999;31:712–717.
Vandervoort J, Soetikno RM, Montes H et al. Accuracy and complication rate of brush cytology from bile duct versus pancreatic duct. Gastrointest Endosc. 1999;49:322–327.
Jailwala J, Fogel EL, Sherman S et al. Triple-tissue sampling at ERCP in malignant biliary obstruction. Gastrointest Endosc. 2000;51:383–390.
Macken E, Drijkoningen M, Van Aken E, Van Steenbergen W. Brush cytology of ductal strictures during ERCP. Acta Gastroenterol Belg. 2000;63:254–259.
Stewart CJ, Mills PR, Carter R et al. Brush cytology in the assessment of pancreatico-biliary strictures: a review of 406 cases. J Clin Pathol. 2001;54:449–455.
de Bellis M, Fogel EL, Sherman S et al. Influence of stricture dilation and repeat brushing on the cancer detection rate of brush cytology in the evaluation of malignant biliary obstruction. Gastrointest Endosc. 2003;58:176–182.
Byrne MF, Gerke H, Mitchell RM et al. Yield of endoscopic ultrasound-guided fine-needle aspiration of bile duct lesions. Endoscopy. 2004;36:715–719.
Eloubeidi MA, Chen VK, Jhala NC et al. Endoscopic ultrasound-guided fine needle aspiration biopsy of suspected cholangiocarcinoma. Clin Gastroenterol Hepatol. 2004;2:209–213.
Fritscher-Ravens A, Broering DC, Knoefel WT et al. EUS-guided fine-needle aspiration of suspected hilar cholangiocarcinoma in potentially operable patients with negative brush cytology. Am J Gastroenterol. 2004;99:45–51.
Khalid A, Pal R, Sasatomi E et al. Use of microsatellite marker loss of heterozygosity in accurate diagnosis of pancreaticobiliary malignancy from brush cytology samples. Gut. 2004;53:1860–1865.
DeWitt J, Misra VL, Leblanc JK, McHenry L, Sherman S. EUS-guided FNA of proximal biliary strictures after negative ERCP brush cytology results. Gastrointest Endosc. 2006;64:325–333.
Fogel EL, deBellis M, McHenry L et al. Effectiveness of a new long cytology brush in the evaluation of malignant biliary obstruction: a prospective study. Gastrointest Endosc. 2006;63:71–77.
Meara RS, Jhala D, Eloubeidi MA et al. Endoscopic ultrasound-guided FNA biopsy of bile duct and gallbladder: analysis of 53 cases. Cytopathology. 2006;17:42–49.
Ornellas LC, Santos Gda C, Nakao FS, Ferrari AP. Comparison between endoscopic brush cytology performed before and after biliary stricture dilation for cancer detection. Arq Gastroenterol. 2006;43:20–23.
Kitajima Y, Ohara H, Nakazawa T et al. Usefulness of transpapillary bile duct brushing cytology and forceps biopsy for improved diagnosis in patients with biliary strictures. J Gastroenterol Hepatol. 2007;22:1615–1620.
Dumonceau JM, Macias Gomez C, Casco C et al. Grasp or brush for biliary sampling at endoscopic retrograde cholangiography? A blinded randomized controlled trial. Am J Gastroenterol. 2008;103:333–340.
Weber A, von Weyhern C, Fend F et al. Endoscopic transpapillary brush cytology and forceps biopsy in patients with hilar cholangiocarcinoma. World J Gastroenterol. 2008;14:1097–1101.
Kim YS, Kim HG, Han J et al. The significance of p53 and K-ras immunocytochemical staining in the diagnosis of malignant biliary obstruction by brush cytology during ERCP. Gut Liver. 2010;4:219–225.
Kawada N, Uehara H, Katayama K et al. Combined brush cytology and stent placement in a single session for presumed malignant biliary stricture. J Gastroenterol Hepatol. 2011;26:1247–1251.
Mohamadnejad M, DeWitt JM, Sherman S et al. Role of EUS for preoperative evaluation of cholangiocarcinoma: a large single-center experience. Gastrointest Endosc. 2011;73:71–78.
Mohammad Alizadeh AH, Mousavi M, Salehi B et al. Biliary brush cytology in the assessment of biliary strictures at a tertiary center in Iran. Asian Pac J Cancer Prev. 2011;12:2793–2796.
Nayar MK, Manas DM, Wadehra V, Oppong KE. Role of EUS/EUS-guided FNA in the management of proximal biliary strictures. Hepatogastroenterology. 2011;58:1862–1865.
Ohshima Y, Yasuda I, Kawakami H et al. EUS-FNA for suspected malignant biliary strictures after negative endoscopic transpapillary brush cytology and forceps biopsy. J Gastroenterol. 2011;46:921–928.
Wright ER, Bakis G, Srinivasan R et al. Intraprocedural tissue diagnosis during ERCP employing a new cytology preparation of forceps biopsy (Smash protocol). Am J Gastroenterol. 2011;106:294–299.
Hartman DJ, Slivka A, Giusto DA, Krasinskas AM. Tissue yield and diagnostic efficacy of fluoroscopic and cholangioscopic techniques to assess indeterminate biliary strictures. Clin Gastroenterol Hepatol. 2012;10:1042–1046.
Kawashima H, Itoh A, Ohno E, Goto H, Hirooka Y. Transpapillary biliary forceps biopsy to distinguish benign biliary stricture from malignancy: how many tissue samples should be obtained? Dig Endosc. 2012;24:22–27.
Krishna N, Tummala P, Labundy J, Agarwal B. EUS guided fine needle aspiration is useful in diagnostic evaluation of indeterminate proximal biliary strictures. Open J. Gastroenterol. 2012;2:33–39.
Nischalke HD, Schmitz V, Luda C et al. Detection of IGF2BP3, HOXB7, and NEK2 mRNA expression in brush cytology specimens as a new diagnostic tool in patients with biliary strictures. PLoS One. 2012;7:e42141.
Lee SJ, Lee YS, Lee MG, Lee SH, Shin E, Hwang JH. Triple-tissue sampling during endoscopic retrograde cholangiopancreatography increases the overall diagnostic sensitivity for cholangiocarcinoma. Gut Liver. 2014;8:669–673.
Nishikawa T, Tsuyuguchi T, Sakai Y et al. Factors affecting the accuracy of endoscopic transpapillary sampling methods for bile duct cancer. Dig Endosc. 2014;26:276–281.
Shieh FK, Luong-Player A, Khara HS et al. Improved endoscopic retrograde cholangiopancreatography brush increases diagnostic yield of malignant biliary strictures. World J Gastrointest Endosc. 2014;6:312–317.
Wakasa T, Inayama K, Honda T, Shintaku M, Okabe Y, Kakudo K. Brushing cytology of the biliary tract: bile juice from the ERCP sheath tube provides cell-rich smear samples. Acta Cytol. 2014;58:398–405.
Weilert F, Bhat YM, Binmoeller KF et al. EUS-FNA is superior to ERCP-based tissue sampling in suspected malignant biliary obstruction: results of a prospective, single-blind, comparative study. Gastrointest Endosc. 2014;80:97–104.
Boldorini R, Paganotti A, Andorno S et al. A multistep cytological approach for patients with jaundice and biliary strictures of indeterminate origin. J Clin Pathol. 2015;68:283–287.
Huang P, Zhang H, Zhang XF, Zhang X, Lyu W, Fan Z. Evaluation of intraductal ultrasonography, endoscopic brush cytology and K-ras, P53 gene mutation in the early diagnosis of malignant bile duct stricture. Chin Med J (Engl). 2015;128:1887–1892.
Nanda A, Brown JM, Berger SH et al. Triple modality testing by endoscopic retrograde cholangiopancreatography for the diagnosis of cholangiocarcinoma. Therap Adv Gastroenterol. 2015;8:56–65.
Sugimoto S, Matsubayashi H, Kimura H et al. Diagnosis of bile duct cancer by bile cytology: usefulness of post-brushing biliary lavage fluid. Endosc Int Open. 2015;3:E323-328.
Chen WM, Wei KL, Chen YS et al. Transpapillary biliary biopsy for malignant biliary strictures: comparison between cholangiocarcinoma and pancreatic cancer. World J Surg Oncol. 2016;14:140.
Naitoh I, Nakazawa T, Kato A et al. Predictive factors for positive diagnosis of malignant biliary strictures by transpapillary brush cytology and forceps biopsy. J Dig Dis. 2016;17:44–51.
Onda S, Ogura T, Kurisu Y et al. EUS-guided FNA for biliary disease as first-line modality to obtain histological evidence. Therap Adv Gastroenterol. 2016;9:302–312.
Walter D, Peveling-Oberhag J, Schulze F et al. Intraductal biopsies in indeterminate biliary stricture: evaluation of histopathological criteria in fluoroscopy- vs. cholangioscopy guided technique. Dig Liver Dis. 2016;48:765–770.
Lee YN, Moon JH, Choi HJ et al. Diagnostic approach using ERCP-guided transpapillary forceps biopsy or EUS-guided fine-needle aspiration biopsy according to the nature of stricture segment for patients with suspected malignant biliary stricture. Cancer Med. 2017;6:582–590.
Costa M, Canena J, Mascarenhas-Lemos L et al. Outcomes of different methods for analysis of biliary brush cytology and of factors associated with positive diagnosis in an age-dependent retrospective review. GE Port J Gastroenterol. 2018;26:5–13.
Inoue T, Kitano R, Kobayashi Y et al. Assessing the diagnostic yield of controllable biopsy-forceps for biliary strictures. Scand J Gastroenterol. 2018;53:598–603.
Kato A, Naitoh I, Miyabe K et al. Differential diagnosis of cholangiocarcinoma and IgG4-related sclerosing cholangitis by fluorescence in situ hybridization using transpapillary forceps biopsy specimens. J Hepatobiliary Pancreat Sci. 2018;25:188–194.
Liew ZH, Loh TJ, Lim TKH et al. Role of fluorescence in situ hybridization in diagnosing cholangiocarcinoma in indeterminate biliary strictures. J Gastroenterol Hepatol. 2018;33:315–319.
Moura DTH, de Moura EGH, Matuguma SE et al. EUS-FNA versus ERCP for tissue diagnosis of suspect malignant biliary strictures: a prospective comparative study. Endosc Int Open. 2018;6:E769–E777.
Ren YC, Huang CL, Chen SM, Zhao QY, Wan XJ, Li BW. Dilation catheter-guided mini-forceps biopsy improves the diagnostic accuracy of malignant biliary strictures. Endoscopy. 2018;50:809–812.
Kobayashi M, Ryozawa S, Araki R et al. Investigation of factors affecting the sensitivity of bile duct brush cytology. Intern Med. 2019;58:329–335.
Nakahara K, Michikawa Y, Morita R et al. Diagnostic Ability of Endoscopic Bile Cytology Using a Newly Designed Biliary Scraper for Biliary Strictures. Dig Dis Sci. 2019;64:241–248.
Onoyama T, Matsumoto K, Takeda Y et al. Endoscopic ultrasonography-guided fine needle aspiration for extrahepatic cholangiocarcinoma: a safe tissue sampling modality. J Clin Med. 2019;8:417.
Yeo SJ, Cho CM, Jung MK, Seo AN, Bae HI. Comparison of the diagnostic performances of same-session endoscopic ultrasound- and endoscopic retrograde cholangiopancreatography-guided tissue sampling for suspected biliary strictures at different primary tumor sites. Korean J Gastroenterol. 2019;73:213–218.
Chang HY, Liu B, Wang YZ et al. Percutaneous transhepatic cholangiography versus endoscopic retrograde cholangiography for the pathological diagnosis of suspected malignant bile duct strictures. Medicine (Baltimore). 2020;99:e19545.
Chapman MH, Tidswell R, Dooley JS et al. Whole genome RNA expression profiling of endoscopic biliary brushings provides data suitable for biomarker discovery in cholangiocarcinoma. J Hepatol. 2012;56:877–885.
Jang SI, Kwon NH, Lim BJ et al. New staining method using methionyl-tRNA synthetase 1 antibody for brushing cytology of bile duct cancer. Gastrointest Endosc. 2020;92:310-319 e316.
Kipp BR, Fritcher EG, Clayton AC et al. Comparison of KRAS mutation analysis and FISH for detecting pancreatobiliary tract cancer in cytology specimens collected during endoscopic retrograde cholangiopancreatography. J Mol Diagn. 2010;12:780–786.
Andresen K, Boberg KM, Vedeld HM et al. Four DNA methylation biomarkers in biliary brush samples accurately identify the presence of cholangiocarcinoma. Hepatology. 2015;61:1651–1659.
Lee JH, Salem R, Aslanian H, Chacho M, Topazian M. Endoscopic ultrasound and fine-needle aspiration of unexplained bile duct strictures. Am J Gastroenterol. 2004;99:1069–1073.
Malikowski T, Levy MJ, Gleeson FC et al. Endoscopic ultrasound/fine needle aspiration is effective for lymph node staging in patients with cholangiocarcinoma. Hepatology. 2020;72:940–948.
Khashab MA, Fockens P, Al-Haddad MA. Utility of EUS in patients with indeterminate biliary strictures and suspected extrahepatic cholangiocarcinoma (with videos). Gastrointest Endosc. 2012;76:1024–1033.
Heimbach JK, Sanchez W, Rosen CB, Gores GJ. Trans-peritoneal fine needle aspiration biopsy of hilar cholangiocarcinoma is associated with disease dissemination. HPB (Oxford). 2011;13:356–360.
El Chafic AH, Dewitt J, Leblanc JK et al. Impact of preoperative endoscopic ultrasound-guided fine needle aspiration on postoperative recurrence and survival in cholangiocarcinoma patients. Endoscopy. 2013;45:883–889.
Author information
Authors and Affiliations
Contributions
Conception and design of the study were contributed by Seung Bae Yoon and Sung-Hoon Moon. Generation, collection, assembly, analysis and/or interpretation of data were contributed by Seung Bae Yoon, Sung-Hoon Moon, Sung Woo Ko, Hyun Lim, and Ho Suk Kang. Drafting of the manuscript was contributed by Seung Bae Yoon and Sung-Hoon Moon. Revision of the manuscript was contributed by Sung Woo Ko, Hyun Lim, Ho Suk Kang, and Jong Hyeok Kim. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no potential conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10620_2021_7138_MOESM1_ESM.docx
Supplementary file 1: Table 1. Preferred reporting items for systematic reviews and meta-analyses checklist. (DOCX 27 kb)
10620_2021_7138_MOESM4_ESM.docx
Supplementary file 4: Table 4. Comparison of pooled sensitivities of brush cytology, forceps biopsy, and EUS-guided sampling among published meta-analyses. (DOCX 18 kb)
10620_2021_7138_MOESM5_ESM.tif
Supplementary file 5: Fig. 1. Pooled rate of adverse events related with tissue acquisition with (A) ERCP-brush, (B) ERCP-biopsy, and (C) EUS-FNA. (TIF 310 kb)
10620_2021_7138_MOESM6_ESM.tif
Supplementary file 6: Fig. 2. Pooled rate of all adverse events with (A) ERCP-brush, (B) ERCP-biopsy, and (C) EUS-FNA. (TIF 297 kb)
10620_2021_7138_MOESM7_ESM.tif
Supplementary file 7: Fig. 3. Pooled diagnostic sensitivities for cholangiocarcinoma according to the obtained number of specimens (< 4 (A) vs. ≥ 4 (B)). (TIF 233 kb)
10620_2021_7138_MOESM8_ESM.tif
Supplementary file 8: Fig. 4. Pooled odds ratio for diagnostic sensitivity of brushing & biopsy method between CCA and malignant biliary strictures other than CCA patients. CCA, cholangiocarcinoma. (TIF 175 kb)
Rights and permissions
About this article
Cite this article
Yoon, S.B., Moon, SH., Ko, S.W. et al. Brush Cytology, Forceps Biopsy, or Endoscopic Ultrasound-Guided Sampling for Diagnosis of Bile Duct Cancer: A Meta-Analysis. Dig Dis Sci 67, 3284–3297 (2022). https://doi.org/10.1007/s10620-021-07138-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-021-07138-4