Abstract
Background
Emerging evidence has suggested that miRNAs are important regulators of intestinal I/R injury, but their function in this context remains elusive.
Aims
To evaluate the role of miR-26b-5p in intestinal I/R injury.
Methods
We utilized in vivo murine models of intestinal I/R and in vitro Mode-K cell-based models of oxygen and glucose deprivation/reperfusion (OGD/R) to examine the function of miR-26b-5p in intestinal I/R injury. The expression of miR-26b-5p in intestinal mucosa and Mode-K cell was detected by RT-PCR. HE staining and Chiu’s score were used to evaluate intestinal mucosa injury severity. Apoptosis was detected by TUNEL stain, flow cytometry, and western blot. TargetScan and StarBase prediction algorithms were applied to predict putative target genes of miR-26b-5p and validated by luciferase reporter analyses.
Results
We found that the expression of miR-26b-5p in intestinal mucosa was markedly decreased during I/R injury. We additionally found miR-26b-5p overexpression to markedly disrupt intestinal I/R- or OGD/R-induced injury in vivo and in vitro, whereas inhibiting this miRNA had an adverse impact and resulted in increased intestinal tissue injury and Mode-K cell damage. From a mechanistic perspective, miR-26b-5p was predicted to target DAPK1, which was related to cellular apoptosis. Luciferase reporter assay results confirmed that miR-26b-5p directly targets DAPK1 in Mode-K cells, thereby suppressing OGD/R-induced cell apoptosis.
Conclusion
Our findings show that miR-26b-5p may prevent intestinal I/R injury via targeting DAPK1 and inhibiting intestinal mucosal cell apoptosis, suggesting that this miRNA may be a viable target for the treatment of intestinal I/R injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intestinal ischemia/reperfusion (I/R) injuries are most often observed in patients suffering from serious conditions including septicemic shock, aortic aneurysm, cardiopulmonary bypass, trauma, or small bowel transplantation [1]. Hypovolemic shock is associated with the preferential shunting of blood flow to the heart and the brain, thus leaving the small intestine at a heightened risk of ischemia [2]. This ischemia and subsequent reperfusion can lead to direct epithelial cell damage and disruption of the integrity of the intestinal barrier, in turn resulting in bacterial translocation, systemic inflammation, and multiple organ failure that can cause high rates of morbidity and mortality [3].
Numerous factors govern intestinal I/R injury development, including the apoptotic death of epithelial cells as well as the excessive production of pro-inflammatory factors, nitric oxide (NO), and reactive oxygen species (ROS) [4,5,6]. Apoptotic cell death, in particular, is thought to be a major regulator of intestinal I/R injury, as it can directly result in the compromise of the intestinal barrier [7]. Caspase proteins are essential effectors and mediators of apoptosis, which can be induced via both the intrinsic and the extrinsic pathways. The exact mechanisms responsible for intestinal epithelial apoptotic cell death in the context of I/R injury, however, remain to be defined [8]. As such, it is essential that additional studies be conducted with the goal of better understanding this condition and thereby identifying novel drug biomarkers that may be targeted in order to effectively prevent or treat intestinal I/R injuries.
MicroRNAs (miRNAs) are non-coding RNAs that are capable of suppressing target mRNA translation or directly mediating target mRNA degradation [9,10,11]. Many miRNAs have been shown to play key roles in the context of intestinal I/R injury, including miR-29b, miR-378, and miR-351 [12,13,14]. We have previously identified miR-26b-5p as a miRNA that is differentially expressed in intestinal mucosal tissues in response to intestinal I/R [14]. Other recent work has suggested that miR-26b-5p dysregulation is also evident in the context of cerebral and myocardial I/R [15]. To date, however, no studies have demonstrated a functional link between miR-26b-5p expression and the incidence of I/R injury.
The serine/threonine kinase death-associated protein kinase 1 (DAPK1) is a calcium/calmodulin-regulated protein that has previously been shown to promote apoptotic cell death via both caspase-independent and -dependent mechanisms [16,17,18]. In the present study, we identified DAPK1 as a putative miR-26b-5p target using a microRNA database (TargetScan http://www.targetscan.org). Based on this predicted relationship, we conducted this analysis with the goal of exploring the functional relationship between miR-26b-5p, DAPK1, and the pathogenesis of intestinal I/R injury.
Materials and Methods
Murine Intestinal I/R Model
Male C57BL/6 J mice (8–10 weeks old) from the Experimental Animals Center at Southern Medical University were used for all animal studies described herein, which were approved by the animal ethics committee of NanFang Hospital based upon National Institutes of Health guidelines. Prior to surgery, animals were fasted for 12 h with free access to water. All mice were then anesthetized via the i.p. injection of 200 mg/kg ketamine and 10 mg/kg xylazine hydrochloride, after which intestinal I/R modeling was conducted as in prior studies [14]. Briefly, we initially exposed the small intestine by generating a ~ 1 cm ventral midline incision (∼1 cm) in the abdomen. A microvessel clip was then used to occlude the superior mesenteric artery (SMA) for 1 h, after which the clip was removed and reperfusion was allowed to proceed for 1, 2, 4, or 12 h. Sham-operated control mice underwent the surgical procedures detailed above, but no occlusion of the SMA was conducted following its exposure. A subcutaneous saline solution injection (50 mL/kg) was administered to all animals after surgery to facilitate resuscitation. Intestinal segments were fixed in 10% neutral formaldehyde, paraffin-embedded for morphological analysis. The intestinal mucosa was washed with cold saline after being scraped off and preserved at –80 °C for detection [14].
Cell Culture and OGD/R Modeling
Mode-K intestinal epithelial cells from the Shanghai Institutes for Biological Sciences (Shanghai, China) were cultured using DMEM containing 10% FBS (Gibco, CA, USA) at 37 °C in a 5% CO2 and 21% O2 incubator (Thermo, CA, USA). OGD conditions were used to mimic intestinal I/R in vitro. This was accomplished by initially growing Mode-K cells to 80% confluence, transferring them to D-Hanks buffer, and culturing them in a 95% N2 and 5% CO2 incubator for 4 h at 37 °C (Thermo). After this OGD induction period, reoxygenation was simulated by restoring these cells to an oxygen-containing incubator for 0, 1, 4, 12, and 24 h as above [19]. Control cells were grown under normoxic conditions for equivalent periods of time.
Tissue Histology
After animals were euthanized, segments of the small intestine were fixed using 4% paraformaldehyde, embedded in paraffin, used to prepare 5 μm sections, and hematoxylin and eosin (H&E)-stained. Two pathologists blinded to study groups then independently evaluated these stained sections, with Chiu’s score which grades from 0 to 8 being used to evaluate injury severity as in prior studies [20]. Criteria of Chiu's score are listed in Table S1.
Cell Viability Analysis
MODE-K cells were plated in 96-well plates (1 × 104/well) overnight in sextuplicate, after which OGD/R experimentation was conducted as above. At appropriate time points, 10 µl of CCK8 solution was added to each well for 2 h at 37℃ after which a POLARstar OPTIMA plate reader (BioRad, CA, USA) was used to assess absorbance at 450 nm.
Flow Cytometry
Mode-K cell apoptosis following OGD/R was assessed via flow cytometry using a commercial kit [21] (KeyGEN, Nanjing, China). Briefly, cells were isolated at appropriate time points, washed with PBS, and stained using Annexin V-FITC/propidium iodide (PI) for 15 min, after which they were evaluated via flow cytometer within 1 h.
Western Blotting
Following total protein extraction from murine intestinal tissues and Mode- cells, Western blotting was conducted as in prior studies. Briefly, we separated 30 μg of protein per sample via 6–20% SDS-PAGE, followed by transfer onto Immobilon-P membranes (Millipore, USA). Blots were blocked using 5% non-fat milk (Biological Technology, China) for 1 h, after which they were probed overnight with anti-DAPK1 (Abcam, MA, USA), anti-cleaved caspase-3 (C-caspase-3) (Abcam, MA, USA), anti-Bcl-2 (Santa Cruz Biotechnology, CA, USA), anti-Bax (Santa Cruz Biotechnology), or anti-GAPDH (Abcam) at 4 °C. Blots were next probed with HRP-linked secondary antibodies, and Pierce ECL Western Blotting Substrate (Thermo Scientific) was then used to visualize protein bands, with GAPDH being used to normalize band intensity values (n = 5).
qRT-PCR
Trizol (Takara, Japan) was used to extract RNA from Mode-K cells and intestinal samples, after which kits from Toyobo and Takara were used to prepare cDNAs for mRNA and miRNA samples, respectively. The expression of miR-26a-5p was evaluated via TaqMan miRNA assay kit (Applied Biosystems, USA) with U6 small nuclear RNA (U6 snRNA) as an internal control, whereas SYBR Green Real-Time PCR Master Mix (ThermoFisher) was used for qRT-PCR. The relative expression level was analyzed using the 2 −△△Ct method.
TUNEL Staining
A TUNEL apoptosis assay kit (Solarbio, Beijing, China) was used based on provided directions to assess apoptosis [21]. A Nikon ECLIPSE microscope (200x) was employed to identify TUNEL-stained nuclei, with the apoptotic rate (%) being given by counting the numbers of positive cells in five randomly chosen areas of view for each sample.
Transfection
Genechem synthesized miR-26b-5p mimics (sense 5′-UUCAAGUAAUUCAGGAUAGGU-3′, antisense 5′-UUCAAGUAAUUCAGGAUAGGU-3′), miR-26b-5p inhibitors (5′-ACCUAUCCUGAAUUACUUGAA-3′), and appropriate negative controls (mimic-NC, sense 5′-UUCUCCGAACGUGUCACGUTT-3′, antisense 5′-ACGUGACACGUUCGGAGAATT-3′ and inhibitor-NC, 5′-CAGUACUUUUGUGUAGUACAA-3′). These constructs were transfected into Mode-K cells plated in 6-well plates using Lipofectamine 3000 (Invitrogen, USA) based on provided directions [22].
For in vivo overexpression and knockdown of miR-26b-5p, agomiR-26b-5p, antagomiR-26b-5p, and appropriate negative controls (agomir-NC and antagomiR-NC) were injected into mice via the tail vein (40 mg/kg, n = 8 per group) for three days. AgomiR-26b-5p and antagomiR-26b-5p were synthesized by RiboBio (Guangzhou, China).
Dual-Luciferase Reporter Assay
TargetScan (http://www.targetscan.org) was used as a means of identifying predicted sites for miR-26b-5p binding within the DAPK1 mRNA. DAPK1 cDNAs that contained either wild-type or mutated versions of this binding site (DAPK1-WT or -MUT) were synthesized and cloned into the pmiRGLO dual-luciferase reporter vector (Genechem, China). Mode-K cells were plated in 24-well plates followed by transfection with appropriate mimic, inhibitor, or negative control constructs for 48 h. A dual-luciferase reporter assay system (Promega, WI, USA) was then used to assess luciferase activity in these cells, with Renilla luciferase serving as a normalization control [23].
Statistical Analysis
Data are means ± SD from triplicate experiments. GraphPad Prism 6.01 (GraphPad Software, USA) was used for all statistical comparisons, with data being compared via Student’s t tests and one-way ANOVAs as appropriate. P < 0.05 was the significance threshold.
Results
I/R and OGD/R Conditions Are Associated with the Induction of Epithelial Damage
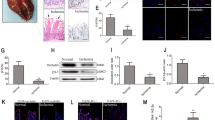
In our murine model of I/R injury, we observed apparent intestinal damage at a range of time points following a 1 h ischemia period and subsequent reperfusion. At 2 h post-reperfusion, there was clear evidence of inflammatory cell infiltration and edema within the mucosal villi, whereas injury severity decreased over time after this 2 h time point as indicated by H&E staining and Chiu’s scores (Fig. 1a, b). In line with these findings, we found that the in vitro OGD/R treatment of Mode-K cells was associated with their decreased viability, especially following 4 h of reoxygenation. (Fig. 1c) TUNEL staining also indicated an increase in the numbers of apoptotic cells in the I/R group in mice and the OGD/R group in Mode-K cells (Fig. 1d–g).
Intestinal I/R damage and cellular apoptosis in mice. a, b H&E staining and histopathological scores (Chiu's score) in mice (n = 8). Male C57BL/6 mice were randomly divided into a sham group and an intestinal I/R groups (60 min of ischemia followed by reperfusion for 1, 2, 4, or 12 h). Scale bar = 100 μm. c Cell viability of Mode-K cells was measured by CCK8 assay. OGD/R injury was induced by depriving cells of oxygen and glucose followed by reperfusion for 0, 4, 8, or 12 h (n = 5). d, f Representative TUNEL staining and apoptotic index values for Mode-K cells that underwent OGD/R (n = 5). Scale bar = 100 μm. e, g Representative TUNEL staining and apoptotic index values for intestinal mucosal tissues in mice following intestinal I/R (n = 8). Scale bar = 100 μm. Data are means ± S.D. h mir-26b-5p expression in the intestinal mucosa of mice after intestinal I/R (n = 8). i mir-26b-5p expression in Mode-K cells after OGD/R (n = 5). *P < 0.05, **P < 0.01 versus the sham or control group; #P < 0.05, ##P < 0.01 versus the 4 h of reoxygenation or 2 h of reperfusion groups
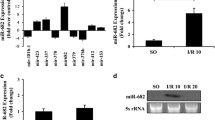
Mir-26b-5p Expression Is Downregulated in Murine Intestines After I/R
RT-PCR was next use to measure the expression of miR-26b-5p in the intestines mucosa of mice subjected to I/R. As shown in Fig. 1h, these miR-26b-5p expression levels were significantly decreased after intestinal I/R. We also determined that the expression levels of miR-26b-5p were markedly downregulated in Mode-k cells after OGD/R, as shown in Fig. 1l.
In Vitro miR-26b-5p Overexpression Is Associated with a Reduction in OGD/R-Induced Cell Damage
In order to evaluate the functional role of miR-26b-5p in the context of intestinal I/R injury, we next transfected miR-26b-5p mimics into Mode-K cells and confirmed successful overexpression of this miRNA via qRT-PCR (Fig. 2a). MiR-26b-5p overexpression resulted in an increase in Mode-K cell viability after OGD/R (Fig. 2b). We additionally determined that miR-26b-5p overexpression suppressed the apoptotic death of these Mode-K cells in response to OGD/R conditions, as determined via TUNEL staining and flow cytometric analyses (Fig. 2c, d). In line with these results, miR-26b-5p overexpression was linked to reductions in the expression of apoptotic markers (cleaved-caspase, Bcl, and Bax) (Fig. 2e).
Overexpression of miR-26b-5p alleviated OGD/R-induced injury. a miR-26b-5p expression levels in Mode-k cells following the transfection of miR-26b-5p mimic or negative control (mimic-NC) constructs (n = 3). b Mode-K cell viability was measured via CCK8 assay. Mode-K cells were transfected with miR-26b-5p mimic or mimic-NC constructs before OGD treatment for 4 h and a 4 h reperfusion period (n = 5). c Representative TUNEL staining and apoptotic index values for Mode-k cells that underwent OGD/R (n = 5). Scale bar = 100 μm. d The rate of Mode-K cell apoptosis was measured by flow cytometry for cells that underwent OGD/R after transfection (n = 5). e The protein levels of C-caspase3 (cleaved-caspase3), Bcl2, and Bax in Mode-k cells that underwent OGD/R after transfection (n = 3). All data are expressed as the mean ± SD. *P < 0.05, **P < 0.01 versus control group
MiR-26b-5p Knockdown Increases the Severity of In Vitro OGD/R-Induced Injury
We next inhibited miR-26b-5p expression in Mode-K cells by transfecting them with a specific inhibitor of this miRNA, with knockdown being confirmed via qRT-PCR (Fig. 3a). Following miR-26b-5p knockdown, we observed reductions in Mode-K cell viability after OGD/R as assessed via CCK-8 assay (Fig. 3b). In line with these results, we found that the apoptosis rates (Fig. 3c, d) and apoptotic marker expression levels in miR-26b-5p-knockdown cells were elevated following OGD/R relative to control cells (Fig. 3e).
Knockdown of miR-26b-5p promoted OGD/R caused injury. a miR-26b-5p expression levels of Mode-k cells after transfection of miR-26b-5p inhibitor and negative control (inhibitor-nc) (n = 3). b Cell viability of Mode-K cell measured by CCK8. Mode-K cells were transfected with miR-26b-5p inhibitor and inhibitor-nc before subjected to depriving the culture media of oxygen and glucose for 4 h and reperfusion for 4 h (n = 5). c, e Representative TUNEL staining and apoptotic index of Mode-k cells underwent OGD/R (n = 5). Scale bar = 100 μm. d, f The apoptosis rate of Mode-k cells underwent OGD/R after transfection was detected by flow cytometry analysis (n = 5). g, h The protein levels of C-caspase3 (cleaved-caspase3), Bcl2 and Bax in Mode-k cells underwent OGD/R after transfection (n = 3). All data are expressed as the mean ± SD. *P < 0.05, **P < 0.01 compared with control group
MiR-26b-5p Overexpression Ameliorates I/R-Induced Intestinal Injury
To further investigate the function of miR-26b-5p in the context of intestinal I/R injury in vivo, we overexpressed this miRNA in C57BL/6 mice via the tail vein injection of miR-26b-5p agomir and confirmed successful overexpression by qRT-PCR (Fig. 4a). The intestinal injury induced by I/R in these mice was significantly attenuated after miR-26b-5p agomir administration as indicated by H&E staining and Chiu’s scores (Fig. 4c). Moreover, fewer apoptotic cells were observed in the intestinal mucosa from the agomir group mice compared to the negative control group (NC) after I/R (Fig. 4e). Consistent with this, pretreatment with miR-26b-5p agomir resulted in a significant reduction in apoptotic marker expression (Fig. 4g).
miR-26b-5p inhibited intestinal I/R injury and cellular apoptosis in vivo. a, b miR-26b-5p expression levels after the administration of agomir, antagomir, and NC constructs. (C and D) H&E staining and histopathological scores (Chiu's score) for mouse intestinal mucosa samples (n = 8). C57BL/6 mice were injected with agomiR-26b-5p, antagomiR-26b-5p, or corresponding NCs (100 μl) via the tail vein (40 mg/kg body weight) for 3 consecutive days and then subjected to intestinal I/R (1 h of ischemia followed by reperfusion for 2 h). Scale bar = 100 μm. (E and F) Representative TUNEL staining and apoptotic index values for mice that underwent I/R (n = 8). Scale bar = 100 μm. g, h The protein levels of C-caspase3 (cleaved-caspase3), Bcl2, and Bax in the intestinal mucosa of mice following I/R (n = 3). All data are expressed as the mean ± SD. *P < 0.05, **P < 0.01 versus control group
MiR-26b-5p Knockdown Aggravates I/R-Induced Intestinal Injury
We further knocked down miR-26b-5p expression in C57BL/6 mice via the intravenous injection of miR-26b-5p antagomir and confirmed successful knockdown via qRT-PCR (Fig. 4b). As shown in Fig. 4d, f, miR-26b-5p knockdown further exacerbated I/R-induced intestinal injury and increased the numbers of apoptotic cells in these animals. Furthermore, miR-26b-5p antagomir administration further increased apoptotic marker expression (Fig. 4h).
MiR-26b-5p Targets DAPK1
We next utilized the StarBase (http://starbase.sysu.edu.cn/mirMrna.php) and TargetScan (http://www.targetscan.org) databases to identify a predicted miR-26b-5p binding site within the DAPK1 3′-UTR. We additionally found that miR-26b-5p overexpression in Mode-K cells was associated with reduced DAPK1 expression, whereas the opposite was observed when this miRNA was knocked down (Fig. 5a). Our in vivo experiments showed similar results as obtained in vitro (Figure S1A and S1B). To confirm that miR-26b-5p targets DAPK1 directly, we transfected Mode-K cells with luciferase reporter constructs containing wild-type or mutated versions of this putative 3′-UTR miRNA binding sequence (DAPK1 3′-UTR-WT or DAPK1 3′-UTR-MUT) (Fig. 5b). We found that miR-26a-5p mimic transfection suppressed and miR-26a-5p inhibitor transfection increased the luciferase activity of WT but not the MUT DANK1 3′-UTR reporter constructs (Fig. 5c, d), suggesting that miR-26a-5p can directly bind to the DAPK1 3′-UTR within Mode-K cells. We next determined whether DAPK1 was responsible for the protective effects induced by miR-26b-5p overexpression. As shown in Fig. 6a, b, miR-26b-5p mimic improved cell viability and reduced apoptotic marker expression under OGD/R conditions, as described above. However, it did not confer such protection after the restoration of the expression of DAPK1, despite the upregulation of miR-26b-5p. Taken together, these data reveal that DAPK1 is required for the regulation of miR-26b-5p-mediated protection against OGD/R injury.
DAPK1 is a miR-26b-5p target gene. a The expression levels of DAPK1 protein after OGD/R injury in Mode-K cells transfected with miR-26b-5p mimic and miR-26b-5p inhibitor constructs (n = 3). b A diagram of the interaction between miR-26b-5p and the 3′-UTR of DAPK1. c, d Relative luciferase expression associated with the DAPK1 3′-UTR in Mode-K cells. Mode-K cells were co-transfected with miR-26-5p mimic, miR-26b-5p inhibitor, or NC constructs as well as DAPK1-WT or DAPK1-MUT reporters (n = 5). All data are expressed as the mean ± SD. *P < 0.05, versus the mimic-NC or inhibitor-NC groups
DAPK1 was responsible for the protective effects induced by miR-26b-5p overexpression. a The viability of Mode-K cells was measured by CCK8 assay. Mode-K cells were co-transfected with miR-26b-5p mimic or DAPK1 plasmid before being subjected to OGD/R (n = 5). b The protein levels of DAPK1, C-caspase3 (cleaved-caspase3), Bcl2, and Bax were measured in Mode-K cells that underwent OGD/R after transfection (n = 3). c Quantitation of the protein levels of DAPK1, C-caspase3 (cleaved-caspase3), Bcl2, and Bax (n = 3). All data are expressed as the mean ± SD. *P < 0.05,**P < 0.01 versus the vector groups
Discussion
Intestinal I/R injuries can arise following shock, trauma, intestinal infarction, and other serious conditions [24]. These injuries can, in turn, result in a breakdown of the intestinal barrier, thereby facilitating bacterial translocation, septicemia, and multi-organ failure leading to death [25]. Herein, we presented two major findings. First, we found that miR-26b-5p was downregulated in the intestinal mucosa during I/R and we confirmed the protective effect of miR-26b-5p against intestinal I/R. Second, we uncovered a novel mechanism by which miR-26b-5p inhibits cellular apoptosis through targeting DAPK1 during intestinal I/R.
I/R-induced epithelial cell apoptosis is one primary mechanism driving I/R injury [26]. Previous work has demonstrated clear roles for miRNAs in the regulation of I/R-associated epithelial cell apoptosis [27]. For example, Wei et al. observed the dysregulation of miR-467, miR-362, miR-379, and miR-668 in a murine model of ischemic acute kidney injury (AKI), and they further found that increasing miR-668 expression was associated with reduced AKI owing to miR-668-mediated targeting of MTP18 and a consequent reduction in mitochondrial fragmentation and apoptotic cell death [28]. Previous work suggests that miR-26b is, along with miR-26a, a member of the miR-26 family of miRNAs [29]. There is clear evidence that miR-26b is dysregulated in a range of disease states, with reductions in circulating miR-26b levels having been observed in myocardial infarction patients, suggesting that this miRNA may protect against adverse cardiomyocyte hypertrophy [30]. Interestingly, Danielsson et al. found that miR-26b inhibited apoptotic cell death in the context of oral lichen planus [31], while other studies found that miR-26b instead promoted apoptotic death in the context of multiple myeloma and hepatocellular carcinoma [32, 33]. As such, the effect of miR-26b-5p on apoptosis can vary in different disease states. To date there have been no prior studies of miR-26b in the context of intestinal I/R. As such, we evaluated the impact of this miRNA on epithelial cell apoptosis in the context of intestinal I/R injury. We determined that miR-26b-5p expression was reduced in murine intestinal epithelial cells in the context of I/R injury, and that overexpressing this miRNA in vitro or in vivo was sufficient to reduce OGD/R or intestinal I/R-induced apoptotic death, whereas miR-26b-5p inhibition exacerbated this apoptotic death. As such, our findings indicate that miR-26b-5p protects against intestinal I/R-induced apoptosis.
Given that miRNAs function at the post-transcriptional level to control target mRNA translation within cells via binding to specific 3′-UTR sequences [34], we utilized a predictive miRNA database (TargetScan) to identify miR-26b-5p targets. DAPK1, which is a positive regulator of apoptotic cell death, was found to be once such putative miR-26b-5p target gene. Activation of DAPK1 in the context of cerebral I/R has been shown to be a key step in the I/R-induced death of neurons. Consistent with these previous results, we confirmed that DAPK1 expression was increased in response to intestinal I/R. We additionally found that miR-26b-5p specifically bound to a DAPK1 3′-UTR sequence and repressed DAPK1 expression in a luciferase reporter assay. These results thus indicated that miR-26b-5p reduces the apoptotic death of intestinal epithelial cells following I/R at least in part via targeting DAPK1.
There are some limitations to the present study. First, downregulation of miR-26b-5p was found in the intestinal mucosa after intestinal I/R, but the detailed mechanisms underlying miR-26b-5p dysregulation were not explored. Second, future studies are needed to investigate whether other targets for miR-26b-5p contribute to the protective effect of miR-26b-5p in intestinal I/R, such as, TCF-4 [35], Gata4 [36], and smad1 [37] which have been reported to involve in cardiac hypertrophy, adipocytic differentiation, and cerebral I/R injury. Third, miR-26b-5p has been reported to be involved in other types of cell death such as autophagy, and as such, more mechanistic studies are needed to fully understand the mechanisms by which miR-26b-5p exert its protective role in the context of intestinal I/R injury.
In conclusion, our findings offer novel evidence suggesting that the miR-26b-5p/DAPK1 signaling pathway is an important regulator of intestinal I/R injury, providing new insights into the pathogenesis of such injuries and suggesting that components of this pathway may be viable therapeutic targets for the prevention or treatment thereof.
References
Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev 2014;94:329–354.
Brand MD, Goncalves RL, Orr AL et al. Suppressors of superoxide-H2O2 production at site IQ of mitochondrial complex I protect against stem cell hyperplasia and ischemia-reperfusion injury. Cell Metabol 2016;24:582–592.
Liu SZ, He XM, Zhang X, Zeng FC, Wang F, Zhou XY. Ischemic preconditioning-induced SOCS-1 protects rat intestinal ischemia reperfusion injury via degradation of TRAF6. Dig Dis Sci 2017;62:105–114. https://doi.org/10.1007/s10620-016-4277-0.
Chassin C, Hempel C, Stockinger S et al. MicroRNA-146a-mediated downregulation of IRAK1 protects mouse and human small intestine against ischemia/reperfusion injury. EMBO Molecular Medicine. 2012;4:1308–1319.
Matsuda A, Yang WL, Jacob A et al. FK866, a visfatin inhibitor, protects against acute lung injury after intestinal ischemia-reperfusion in mice via NF-kappaB pathway. Ann Surg 2014;259:1007–1017.
Zhang XY, Liu ZM, Wen SH et al. Dexmedetomidine administration before, but not after, ischemia attenuates intestinal injury induced by intestinal ischemia-reperfusion in rats. Anesthesiology 2012;116:1035–1046.
Grootjans J, Hodin CM, de Haan JJ et al. Level of activation of the unfolded protein response correlates with Paneth cell apoptosis in human small intestine exposed to ischemia/reperfusion. Gastroenterology 2011;140:e523.
Matsuo S, Yang WL, Aziz M, Jacob A, Wang P. Cyclic arginine-glycine-aspartate attenuates acute lung injury in mice after intestinal ischemia/reperfusion. Crit Care (London, England). 2013;17:R19.
Huang W, Gu J, Tao T, Zhang J, Wang H, Fan Y. MiR-24-3p Inhibits the Progression of Pancreatic Ductal Adenocarcinoma Through LAMB3 Downregulation. Frontiers in Oncology. 2019;9:1499.
Yang LW, Wu XJ. miR-155 increases stemness and decitabine resistance in triple-negative breast cancer cells by inhibiting TSPAN5. Mol Carcinog 2020;59:447–461.
Shen XF, Cheng Y, Dong QR, Zheng MQ. MicroRNA-675-3p regulates IL-1beta-stimulated human chondrocyte apoptosis and cartilage degradation by targeting GNG5. Biochem Biophys Res Commun 2020;523:46–53.
Hu Y, Tao X, Han X et al. MicroRNA-351-5p aggravates intestinal ischemia/reperfusion injury through the targeting of MAPK13 and Sirtuin-6. Br J Pharmacol 2018;175:3594–3609.
Dai Y, Mao Z, Han X et al. MicroRNA-29b-3p reduces intestinal ischaemia/reperfusion injury via targeting of TNF receptor-associated factor 3. Br J Pharmacol 2019;176:3264–3278.
Li Y, Wen S, Yao X et al. MicroRNA-378 protects against intestinal ischemia/reperfusion injury via a mechanism involving the inhibition of intestinal mucosal cell apoptosis. Cell Death Dis 2017;8:e3127.
Li G, Xiao L, Qin H et al. Exosomes-carried microRNA-26b-5p regulates microglia M1 polarization after cerebral ischemia/reperfusion. Cell Cycle (Georgetown, Tex.) 2020;19:1022–1035.
Chen D, Mei Y, Kim N et al. Melatonin directly binds and inhibits death-associated protein kinase 1 function in Alzheimer’s disease. J Pineal Res 2020;69:e12665.
Farag AK, Roh EJ. Death-associated protein kinase (DAPK) family modulators: Current and future therapeutic outcomes. J Pineal Res 2019;39:349–385.
Singh P, Ravanan P, Talwar P. Death associated protein kinase 1 (DAPK1): a regulator of apoptosis and autophagy. Med Res Rev 2016;9:46.
Liu ZM, Zhang XY, Chen J, Shen JT, Jiang ZY, Guan XD. Terlipressin protects intestinal epithelial cells against oxygen-glucose deprivation/re-oxygenation injury via the phosphatidylinositol 3-kinase pathway. Exp Therapeut Med 2017;14:260–266.
Arisue A, Shimojima N, Tomiya M et al. Effect of an omega-3 lipid emulsion in reducing oxidative stress in a rat model of intestinal ischemia-reperfusion injury. Pediatric Surg Int 2012;28:913–918.
Kabakov AE, Gabai VL. Cell death and survival assays. Methods Mol Bio (Clifton, N.J.) 2018;1709:107–127.
Cheng L, Deng G, Mou T et al. Circular RNA circRHOBTB3 acts as a sponge for miR-654-3p inhibiting gastric cancer growth. Cancer Med 2020;39:1.
Chen D, Guo Y, Chen Y et al. LncRNA growth arrest-specific transcript 5 targets miR-21 gene and regulates bladder cancer cell proliferation and apoptosis through PTEN. Cancer Med 2020;9:2846–2858.
Koike Y, Li B, Lee C et al. The intestinal injury caused by ischemia-reperfusion is attenuated by amniotic fluid stem cells via the release of tumor necrosis factor-stimulated gene 6 protein. FASEB J 2020;34:6824–6836.
Li Y, Feng D, Wang Z et al. Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ 2019;26:2284–2299.
Chen SY, Zhang HP, Li J, Shi JH. Tripartite motif-containing 27 attenuates liver ischemia/reperfusion injury by suppressing TAK1 via TAB2/3 degradation. Hepatology 2020;73:738–758.
Li M, Ding W, Tariq MA et al. A circular transcript of ncx1 gene mediates ischemic myocardial injury by targeting miR-133a-3p. Theranostics 2018;8:5855–5869.
Chun N, Coca SG, He JC. A protective role for microRNA-688 in acute kidney injury. J Clin Investig 2018;128:5216–5218.
Chen CY, Chang JT, Ho YF, Shyu AB. MiR-26 down-regulates TNF-alpha/NF-kappaB signalling and IL-6 expression by silencing HMGA1 and MALT1. Nucleic Acids Res 2016;44:3772–3787.
Jakob P, Kacprowski T, Briand-Schumacher S et al. Profiling and validation of circulating microRNAs for cardiovascular events in patients presenting with ST-segment elevation myocardial infarction. European Heart J 2017;38:511–515.
Danielsson K, Ebrahimi M, Wahlin YB, Nylander K, Boldrup L. Increased levels of COX-2 in oral lichen planus supports an autoimmune cause of the disease. J Eur Acad Dermatol Venereol JEADV 2012;26:1415–1419.
Jia CM, Tian YY, Quan LN, Jiang L, Liu AC. miR-26b-5p suppresses proliferation and promotes apoptosis in multiple myeloma cells by targeting JAG1. Pathol Res Pract 2018;214:1388–1394.
Lin Y, Jian Z. Long non-coding RNA DLGAP1-AS1 facilitates tumorigenesis and epithelial-mesenchymal transition in hepatocellular carcinoma via the feedback loop of miR-26a/b-5p/IL-6/JAK2/STAT3 and Wnt/beta-catenin pathway. Cell Death Dis 2020;11:34.
Forero A, So L, Savan R. Re-evaluating strategies to define the immunoregulatory roles of miRNAs. Trends Immunol 2017;38:558–566.
Luo Y, Ji H, Cao Y, Ding X, Li M. miR-26b-5p/TCF-4 controls the adipogenic differentiation of human adipose-derived mesenchymal stem cells. Cell Transplant 2020;29:963689720934418.
Han M, Yang Z, Sayed D et al. GATA4 expression is primarily regulated via a miR-26b-dependent post-transcriptional mechanism during cardiac hypertrophy. Cell Transplant 2012;93:645–654.
Shangguan Y, Han J, Su H. GAS5 knockdown ameliorates apoptosis and inflammatory response by modulating miR-26b-5p/Smad1 axis in cerebral ischaemia/reperfusion injury. Behav Brain Res 2020;379:112370.
Funding
This study was supported by Grant No. 81671955 from the National Natural Science Foundation of China (to Dr. Ke-Xuan Liu) and Grant No. 81730058 from the Key Program of the National Natural Science Foundation of China (to Dr. Ke-Xuan Liu).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the Ethics Committee of NanFang Hospital.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Zhou, B., Zhang, W., Yan, Z. et al. MicroRNA-26b-5p Targets DAPK1 to Reduce Intestinal Ischemia/Reperfusion Injury via Inhibition of Intestinal Mucosal Cell Apoptosis. Dig Dis Sci 67, 1794–1805 (2022). https://doi.org/10.1007/s10620-021-06975-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-021-06975-7