Abstract
Background
The Gemini trial failed to detect a significant difference in response rate for patients with ulcerative colitis (UC) randomized to standard (every 8 week) vedolizumab dosing vs escalated (every 4 week) dosing. Subsequent real-world data imply the Gemini trial design may have obscured a benefit of escalated dosing.
Aims
We investigated outcomes after vedolizumab dose escalation for patients with UC. We also explored potential clinical predictors of dose escalation requirement.
Methods
In this retrospective study, we included patients with UC who received vedolizumab between 1/2017-1/2019. We compared rates of clinical response (decrease in partial Mayo score by ≥ 2) and remission (partial Mayo < 2) for standard vs escalated dosing.
Results
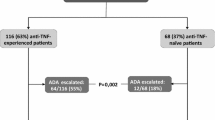
Among the 90 patients reviewed, 52 achieved and maintained remission on standard dosing. The average time to remission with standard dosing was 33.3 ± 6.6 weeks. After an average of 56.3 ± 7.4 weeks standard dosing, 24 patients (22 “partial responders” and 2 “non-responders”) were dose-escalated. Of the 22 “partial responders” dose-escalated, 10 (45%) achieved remission, 10 (45%) achieved further improvement. Neither “non-responder” demonstrated further clinical benefit. Prior anti-tumor necrosis factor (anti-TNF) biologic exposure predicted dose escalation requirement (p = 0.008). Patients requiring dose escalation had more severe disease at baseline as measured by both full Mayo (p = 0.009) and partial Mayo scores (p = 0.01).
Conclusions
We show dose escalation benefited patients with UC who exhibit a “partial response” to standard dosing. Early vedolizumab dose escalation should be considered in both patients with severe disease and those with prior anti-TNF experience.





Similar content being viewed by others
References
Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476. https://doi.org/10.1056/NEJMoa050516.
Colombel JF, Rutgeerts P, Reinisch W, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011;141:1194–1201. https://doi.org/10.1053/j.gastro.2011.06.054.
Papamichael K, Lin S, Moore M, Papaioannou G, Sattler L, Cheifetz AS. Infliximab in inflammatory bowel disease. Ther Adv Chronic Dis. 2019;10:2040622319838443. https://doi.org/10.1177/2040622319838443.
Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–1395. https://doi.org/10.1056/NEJMoa0904492.
Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699–710. https://doi.org/10.1056/NEJMoa1215734.
Colombel JF, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut. 2017;66:839–851. https://doi.org/10.1136/gutjnl-2015-311079.
Chaparro M, Garre A, Ricart E, et al. Short and long-term effectiveness and safety of vedolizumab in inflammatory bowel disease: results from the ENEIDA registry. Aliment Pharmacol Ther. 2018;48:839–851. https://doi.org/10.1111/apt.14930.
Vermeire S, Lukas M, Magro F, et al. Vedolizumab efficacy, safety, and pharmacokinetics with reduced frequency of dosing from every 4 weeks to every 8 weeks in patients with Crohn’s disease or ulcerative colitis. J Crohns Colitis. 2020. https://doi.org/10.1093/ecco-jcc/jjaa027.
Chan W, Lynch N, Bampton P, et al. Entyvio lengthen dose-interval study: lengthening vedolizumab dose interval and the risk of clinical relapse in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2018;30:735–740. https://doi.org/10.1097/MEG.0000000000001150.
Feagan BG, Lasch K, Lissoos T, et al. Rapid response to vedolizumab therapy in biologic-naive patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2019;17:130-8e7. https://doi.org/10.1016/j.cgh.2018.05.026.
Rosario M, French JL, Dirks NL, et al. Exposure-efficacy relationships for vedolizumab induction therapy in patients with ulcerative colitis or Crohn’s disease. J Crohns Colitis. 2017;11:921–929. https://doi.org/10.1093/ecco-jcc/jjx021.
Berends SE, Strik AS, Lowenberg M, D’Haens GR, Mathot RAA. Clinical pharmacokinetic and pharmacodynamic considerations in the treatment of ulcerative colitis. Clin Pharmacokinet. 2019;58:15–37. https://doi.org/10.1007/s40262-018-0676-z.
Rosario M, Dirks NL, Gastonguay MR, et al. Population pharmacokinetics-pharmacodynamics of vedolizumab in patients with ulcerative colitis and Crohn’s disease. Aliment Pharmacol Ther. 2015;42:188–202. https://doi.org/10.1111/apt.13243.
Singh S, Dulai PS, Vande Casteele N, et al. Systematic review with meta-analysis: association between vedolizumab trough concentration and clinical outcomes in patients with inflammatory bowel diseases. Aliment Pharmacol Ther. 2019;50:848–857. https://doi.org/10.1111/apt.15484.
Dulai PS, Singh S, Casteele NV, et al. Development and validation of clinical scoring tool to predict outcomes of treatment with vedolizumab in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2020. https://doi.org/10.1016/j.cgh.2020.02.010.
Reinisch W, Bressler B, Curtis R, et al. Fecal calprotectin responses following induction therapy with vedolizumab in moderate to severe ulcerative colitis: a post hoc analysis of GEMINI 1. Inflamm Bowel Dis. 2019;25:803–810. https://doi.org/10.1093/ibd/izy304.
Acknowledgments
The project described was supported by the NIH National Center for Advancing Translational Sciences through Grant Number UL1TR001998. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We would also like to thank Nancy Mannon RPh, for her assistance with compiling patients meeting eligibility criteria for study analysis.
Funding
CP is supported by the National Institutes of Health, Grant TL1TR001997 of CCTS funding. The authors’ work is also supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number R21DK118954. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This work was supported in part by Merit Review Award# I01CX001353 to TAB from the United States (U.S.) Department of Veterans Affairs Laboratory Research and Development Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Barrett has consulted and received honoraria for speaker’s bureau activities for Takeda and AbbVie pharmaceutical companies.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Perry, C., Fischer, K., Elmoursi, A. et al. Vedolizumab Dose Escalation Improves Therapeutic Response in a Subset of Patients with Ulcerative Colitis. Dig Dis Sci 66, 2051–2058 (2021). https://doi.org/10.1007/s10620-020-06486-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-020-06486-x