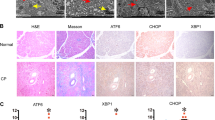
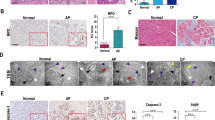
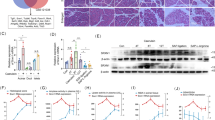
Abstract
Background and Aims
Acute pancreatitis (AP) is a severe pancreatic disorder that remains associated with high mortality due to a lack of effective drugs and management strategies. This study aimed to investigate the molecular pathogenic mechanisms of AP involving p53 and endoplasmic reticulum (ER) stress pathways.
Methods
Expression of PRSS1 and p53 in human AP tissues was detected by immunohistochemistry and Western blotting. AP was induced with caerulein in humanized PRSS1 transgenic mice, and its severity was verified by histological imaging, evaluation of edema, serum amylase, and trypsin activity assays. A transferase-mediated d-UTP nick end-labeling assay was performed to evaluate acinar cell apoptosis associated with AP. The expression of ER stress genes was assessed by quantitative RT-PCR (qRT-PCR) and Western blotting.
Results
PRSS1 and p53 were highly expressed in human AP tissues. Expression of human PRSS1 in caerulein-treated mice induced significant acinar cell apoptosis and AP progression. P53 knockout significantly suppressed AP progression in humanized PRSS1 transgenic mice. The ER stress pathway was activated by PRSS1 and mediated the progression of AP in mouse pancreatic tissues. Application of a p53 inhibitor effectively ameliorated caerulein-induced AP in PRSS1 transgenic mice, while a p53 activator promoted the progression of AP.
Conclusion
P53, which was activated by the ER stress pathway, promoted the progression of AP in mice expressing PRSS1 by inducing acinar cell apoptosis.






Similar content being viewed by others
References
Lankisch PG, Apte M, Banks PA. Acute pancreatitis. Lancet. 2015;386:85-96.
Waldthaler A, Schütte K, Malfertheiner P. Causes and mechanisms in acute pancreatitis. Dig Dis. 2010;28:364-372.
Rajinder D, Sah RP, Vikas D, Loveena R, Rupjoyti T, Pramod G, Saluja AK. Intra-acinar trypsinogen activation mediates early stages of pancreatic injury but not inflammation in mice with acute pancreatitis. Gastroenterology. 2011;141:2210-2217
Whitcomb DC, Gorry MC, Preston RA, Furey W, Sossenheimer MJ, Ulrich CD, Martin SP, Gates LK, Amann ST, Toskes PP. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996;14:141.
Balázs Csaba N, Miklós ST. Human cationic trypsinogen (PRSS1) variants and chronic pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2014;306:G466-73.
Sánchez-Ramírez CA, Flores-Martínez SE, García-Zapién AG, Montero-Cruz SA, Larrosa-Haro A, Sánchez-Corona J. Screening of R122H and N29I mutations in the PRSS1 gene and N34S mutation in the SPINK1 gene in Mexican pediatric patients with acute and recurrent pancreatitis. Pancreas. 2012;41:707.
Sebastian G, Jaroslaw D, Yan L, Lilian T, Jun C, Woojin L, Longnecker DS, Logsdon CD, Baoan J. Intracellular activation of trypsinogen in transgenic mice induces acute but not chronic pancreatitis. Gut. 2011;60:1379-88.
Athwal T, Huang W, Mukherjee R, Latawiec D, Chvanov M, Clarke R, Smith K, Campbell F, Merriman C, Criddle D. Expression of human cationic trypsinogen (PRSS1) in murine acinar cells promotes pancreatitis and apoptotic cell death. Cell Death Dis. 2014;5:e1165.
Guillermo G, Englander EW, Guiyun W, Greeley GH. Increased expression of hypoxia-inducible factor-1alpha, p48, and the Notch signaling cascade during acute pancreatitis in mice. Pancreas. 2004;28:58-64.
Yuji N, Jae Hyuk D, Jingzhen Y, Odinokova IV, Olga M, Gukovskaya AS, Pandol SJ. Inflammatory cells regulate p53 and caspases in acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2010;298:G92-100.
Jiang PH, Motoo Y, Iovanna JL, Pébusque MJ, Xie MJ, Okada G, Sawabu N. Tumor protein p53-induced nuclear protein 1 (TP53INP1) in spontaneous chronic pancreatitis in the WBN/Kob rat: drug effects on its expression in the pancreas. J Pancreas. 2004;5:205.
Chen P, Huang L, Zhang Y, Qiao M, Yuan Y. SiRNA-mediated PIAS1 silencing promotes inflammatory response and leads to injury of cerulein-stimulated pancreatic acinar cells via regulation of the P38MAPK signaling pathway. Int J Mol Med. 2010;26:619-626.
Ouyang J, Zhang ZH, Zhou YX, Niu WC, Zhou F, Shen CB, Chen RG, Li X. Up-regulation of Tight-Junction Proteins by p38 Mitogen-Activated Protein Kinase/p53 Inhibition Leads to a Reduction of Injury to the Intestinal Mucosal Barrier in Severe Acute Pancreatitis. Pancreas. 2016;45:1136.
Chen J, Chen J, Wang X, Wang C, Cao W, Zhao Y, Zhang B, Cui M, Shi Q, Zhang G. Ligustrazine alleviates acute pancreatitis by accelerating acinar cell apoptosis at early phase via the suppression of p38 and Erk MAPK pathways. Biomed Pharmacother. 2016;82:1-7.
Shen A, Kim HJ, Oh GS, Lee SB, Lee SH, Pandit A, Khadka D, Choe SK, Kwak SC, Yang SH. NAD (+) augmentation ameliorates acute pancreatitis through regulation of inflammasome signalling. Sci Rep. 2017;7:3006.
Fukuda N, Saitoh M, Kobayashi N, Miyazono K. Execution of BMP-4-induced apoptosis by p53-dependent ER dysfunction in myeloma and B-cell hybridoma cells. Oncogene. 2006;25:3509-3517.
Nikolina D, Ioulia C, Elena F, Papavassiliou AG, Hippokratis K. p53 antagonizes the unfolded protein response and inhibits ground glass hepatocyte development during endoplasmic reticulum stress. Exp Biol Med. 2012;237:1173-80.
Thomas SE, Elke M, Adriana OE, Dalton LE, Wout EFAV, Elizabeth L, Crowther DC, Lomas DA, Marciniak SJ. p53 and translation attenuation regulate distinct cell cycle checkpoints during endoplasmic reticulum (ER) stress. J Biol Chem. 2013;288:7606-7617.
Kubisch CH, Maria Dolors S, Thiruvengadam A, Ernst SA, Williams JA, Logsdon CD. Early activation of endoplasmic reticulum stress is associated with arginine-induced acute pancreatitis. A J Physiol Gastrointest Liver Physiol. 2006;291:G238-45.
Cai Y, Shen Y, Xu G, Tao R, Yuan W, Huang Z, Zhang D. TRAM1 protects AR42J cells from caerulein-induced acute pancreatitis through ER stress-apoptosis pathway. In Vitro Cell Dev Biol Anim. 2016;52:530-6.
Zhou L, Tan JH, Cao RC, Xu J, Chen XM, Qi ZC, Zhou SY, Li SB, Mo QX, Li ZW, Zhang GW. ATF6 regulates the development of chronic pancreatitis by inducing p53-mediated apoptosis. Cell Death Dis. 2019;10(9):662.
Binker MG, Daniel R, Gaisano HY, Cosen-Binker LI. ER stress-associated CTRC mutants decrease stimulated pancreatic zymogen secretion through SIRT2-mediated microtubule dysregulation. Biochem Biophys Res Commun. 2015;463:329-335.
Lerch MM1, Gorelick FS. Models of acute and chronic pancreatitis. Gastroenterology. 2013;144:1180-1193.
Barlass U, Dutta R, Cheema H, George J, Sareen A, Dixit A, Yuan Z, Giri B, Meng J, Banerjee S, Banerjee S, Dudeja V, Dawra RK, Roy S, Saluja AK. Morphine worsens the severity and prevents pancreatic regeneration in mouse models of acute pancreatitis. Gut. 2018;67:600-602.
Thomas DDH, Kaspar KM, Taft WB, Ning W, Rodenkirch LA, Groblewski GE. Identification of annexin VI as a Ca2+-sensitive CRHSP-28-binding protein in pancreatic acinar cells. J Biol Chem. 2002;277:35496-35502.
Wisner J, Green D, Ferrell L, Renner I. Evidence for a role of oxygen derived free radicals in the pathogenesis of caerulein induced acute pancreatitis in rats. Gut. 1988;29:1516-1523.
Hofbauer B, Saluja AK, Lerch MM, Bhagat L, Bhatia M, Lee HS, Frossard JL, Adler G, Steer ML. Intra-acinar cell activation of trypsinogen during caerulein-induced pancreatitis in rats. Am J Physiol. 1998;275:G352-362.
Niederau C, Ferrell LD, Grendell JH. Caerulein-induced acute necrotizing pancreatitis in mice: protective effects of proglumide, benzotript, and secretin. Gastroenterology. 1985;88:1192-1204.
Cregan SP, Maclaurin JG, Craig CG, Robertson GS, Nicholson DW, Park DS, Slack RS. Bax-dependent caspase-3 activation is a key determinant in p53-induced apoptosis in neurons. J Neurosci Off J Soc Neurosci. 1999;19:7860.
Ding HF, Lin YL, Mcgill G, Juo P, Zhu H, Blenis J, Yuan J, Fisher DE. Essential role for caspase-8 in transcription-independent apoptosis triggered by p53. J Biol Chem. 2000;275:38905-38911.
Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B. A model for p53-induced apoptosis. Nature. 1997;389:300-305.
Casey G, Yamanaka Y, Friess H, Kobrin MS, Lopez ME, Buchler M, Beger HG, Korc M. p53 Mutations are common in pancreatic cancer and are absent in chronic pancreatitis. Cancer Lett. 1993;69:151-160.
Jeong S, Lee DH, Lee JI, Lee JW, Kwon KS, Kim PS, Kim HG, Yong WS, Kim YS, Kim YB. Expression of Ki-67, p53, and K-ras in chronic pancreatitis and pancreatic ductal adenocarcinoma. World J Gastroentero. 2005;11:6765-6769.
Arturo R, Antonina B, Roberto G, Patrizia D, Rita F, Maurizio C, Luigi P, Gianfranco P, Maria Felice B. p53 Mediates the accelerated onset of senescence of endothelial progenitor cells in diabetes. J Biol Chem. 2006;281(7):4339-47.
Yoshida H. ER stress and diseases. Febs J. 2007;274:630-658.
Like Q, Shirley H, Dionissios B, Ana-Maria RE, Olivier P, Maria H, Costas K, Yoichi T, Akihiko Y, Koromilas AE. Endoplasmic reticulum stress induces p53 cytoplasmic localization and prevents p53-dependent apoptosis by a pathway involving glycogen synthase kinase-3beta. Genes Dev. 2004;18:261-77.
Lin WC, Chuang YC, Chang YS, Lai MD, Teng YN, Su IJ, Wang CC, Lee KH, Hung JH. Endoplasmic reticulum stress stimulates p53 expression through NF-κB activation. PLoS ONE. 2012;7:e39120.
Wu JS, Li WM, Chen YN, Zhao Q, Chen QF. Endoplasmic reticulum stress is activated in acute pancreatitis. J Dig Dis. 2016;17:295-303.
Funding
The article is supported by Guangdong Science and Technology Planning Project (2019A030317018) & Scientific Research Startup Program of Southern Medical University by High-level University Construction Funding of Guangdong Provincial Department of Education (CX2018N012) & Clinical Research Program of Nanfang Hospital, Southern Medical University (2018CR046) & Clinical Research Startup Program of Southern Medical University by High-level University Construction Funding of Guangdong Provincial Department of Education (LC2016PY011).
Author information
Authors and Affiliations
Contributions
GZ conceived and designed the study, and critically revised the manuscript. LZ and JT performed the experiments, analyzed the data, and drafted the manuscript. WZ, JX, SR, ZL, and XC participated in study design, study implementation, and manuscript revision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, L., Tan, Jh., Zhou, Wy. et al. P53 Activated by ER Stress Aggravates Caerulein-Induced Acute Pancreatitis Progression by Inducing Acinar Cell Apoptosis. Dig Dis Sci 65, 3211–3222 (2020). https://doi.org/10.1007/s10620-020-06052-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-020-06052-5