Abstract
Background
Data on real-life patterns of biologic use for inflammatory bowel disease (IBD) are scarce.
Aims
We aimed to examine the patterns of biologic use and the factors associated with non-persistence and switching of biologics in Korean IBD patients.
Methods
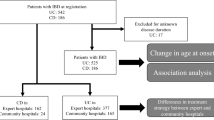
Using National Health Insurance claims, we collected data on patients who were diagnosed with IBD and exposed to biologics between 2010 and 2016.
Results
Among 1838 patients with Crohn’s disease (CD), 1237 and 601 started with infliximab and adalimumab, respectively. Among 1125 patients with ulcerative colitis (UC), 774, 294, and 57 initiated infliximab, adalimumab, and golimumab, respectively. Rates of non-persistence and switching were higher in UC than in CD. One- and 3-year non-persistence rates were 14.2% and 26.5% in CD and 35.4% and 53.4% in UC, respectively. One- and 3-year switching rates were 3.7% and 10.1% in CD and 15.6% and 22.0% in UC, respectively. In both CD and UC, infliximab and adalimumab initiators showed similar persistence rates, whereas adalimumab initiators had a higher risk of switching than infliximab initiators. In UC, golimumab initiators had a higher risk of non-persistence and switching than infliximab initiators. Steroid use at biologic initiation was associated with an increased risk of non-persistence and switching in both CD and UC. UC patients who started biologic treatment at tertiary hospitals were more likely to continue treatment than those who started at general hospitals/community hospitals/clinics.
Conclusions
In real-world clinical practice settings, discontinuation of biologics occurred frequently in IBD patients, and switching of biologics was common in UC patients.


Similar content being viewed by others
References
Park JJ, Yang SK, Ye BD, et al. Second Korean guidelines for the management of Crohn’s disease. Intest Res. 2017;15:38–67.
Choi CH, Moon W, Kim YS, et al. Second Korean guidelines for the management of ulcerative colitis. Intest Res. 2017;15:7–37.
Ng WK, Wong SH, Ng SC. Changing epidemiological trends of inflammatory bowel disease in Asia. Intest Res. 2016;14:111–119.
Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54.
Yen HH, Weng MT, Tung CC, et al. Epidemiological trend in inflammatory bowel disease in Taiwan from 2001 to 2015: a nationwide populationbased study. Intest Res. 2019;17:54–62.
Danese S, Vuitton L, Peyrin-Biroulet L. Biologic agents for IBD: practical insights. Nat Rev Gastroenterol Hepatol. 2015;12:537–545.
Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549.
Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476.
Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323–333.
Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142:257–265.
Ha C, Ullman TA, Siegel CA, et al. Patients enrolled in randomized controlled trials do not represent the inflammatory bowel disease patient population. Clin Gastroenterol Hepatol. 2012;10:1002–1007.
Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11:44–47.
Patel H, Lissoos T, Rubin DT. Indicators of suboptimal biologic therapy over time in patients with ulcerative colitis and Crohn’s disease in the United States. PLoS One. 2017;12:e0175099.
Khan S, Rupniewska E, Neighbors M, et al. Real-world evidence on adherence, persistence, switching and dose escalation with biologics in adult inflammatory bowel disease in the United States: a systematic review. J Clin Pharm Ther. 2019;44:495–507.
Park SH, Kim YM, Yang SK, et al. Clinical features and natural history of ulcerative colitis in Korea. Inflamm Bowel Dis. 2007;13:278–283.
Ng SC, Tsoi KK, Kamm MA, et al. Genetics of inflammatory bowel disease in Asia: systematic review and meta-analysis. Inflamm Bowel Dis. 2012;18:1164–1176.
Wei SC. Differences in the public medical insurance systems for inflammatory bowel disease treatment in Asian countries. Intest Res. 2016;14:218–223.
Jung YS, Han M, Kim WH, et al. Incidence and clinical outcomes of inflammatory bowel disease in South Korea, 2011-2014: a nationwide population-based study. Dig Dis Sci. 2017;62:2102–2112.
Jung YS, Han M, Park S, et al. Cancer risk in the early stages of inflammatory bowel disease in Korean patients: a nationwide population-based study. J Crohns Colitis. 2017;11:954–962.
Han M, Jung YS, Cheon JH, et al. Regional variations in the use of biologics and immunomodulators among Korean patients with inflammatory bowel diseases. J Gastroenterol Hepatol. 2019;34:1166–1174.
Ooi CJ, Hilmi I, Banerjee R, et al. Best practices on immunomodulators and biologic agents for ulcerative colitis and Crohn’s disease in Asia. Intest Res. 2019;17:285–310.
Null KD, Xu Y, Pasquale MK, et al. Ulcerative Colitis treatment patterns and cost of care. Value Health. 2017;20:752–761.
Chen C, Hartzema AG, Xiao H, et al. Real-world pattern of biologic use in patients with inflammatory bowel disease: treatment persistence, switching, and importance of concurrent immunosuppressive therapy. Inflamm Bowel Dis. 2019;25:1417–1427.
Choi CH, Song ID, Kim YH, et al. Efficacy and safety of infliximab therapy and predictors of response in Korean patients with Crohn’s disease: a nationwide multicenter study. Yonsei Med J. 2016;57:1376–1385.
Lee KM, Jeen YT, Cho JY, et al. Efficacy, safety, and predictors of response to infliximab therapy for ulcerative colitis: a Korean multicenter retrospective study. J Gastroenterol Hepatol. 2013;28:1829–1833.
Desai A, Zator ZA, de Silva P, et al. Older age is associated with higher rate of discontinuation of anti-TNF therapy in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:309–315.
Paul S, Roblin X. Letter: immunogenicity of anti-TNF in elderly IBD patients. Aliment Pharmacol Ther. 2019;50:336.
Kim ES, Kim KO, Jang BI, et al. Factors contributing to the preference of Korean patients with Crohn’s disease when selecting an anti-tumor necrosis factor agent (CHOICE study). Gut Liver. 2016;10:391–398.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jung, Y.S., Han, M., Park, S. et al. Biologic Use Patterns and Predictors for Non-persistence and Switching of Biologics in Patients with Inflammatory Bowel Disease: A Nationwide Population-Based Study. Dig Dis Sci 65, 1436–1444 (2020). https://doi.org/10.1007/s10620-019-05867-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-019-05867-1