Abstract
Background
There are many studies on submucosal injection materials, but their clinical use is restricted for various reasons. The objective of this study was to compare the feasibility and safety of injected EndoClot®SIS polysaccharide as a submucosal injection material (SFC) in ESD in the pig stomach to that of injected sigMAVisc™ or Eleview™.
Methods
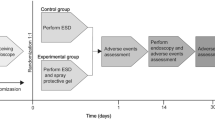
Four pig stomachs were used for the ex vivo study. Eighteen pigs were used for the in vivo study. In the ex vivo study, four injections were made in the gastric submucosa to induce submucosal uplift and extend its duration. Tissue change was observed. The in vivo study was performed in 2 steps. First, 3 injections were made in the esophageal mucosa to induce submucosal uplift and extend its duration. Histological change was observed. Second, ESD was performed in the stomach by injecting EndoClot®SIS polysaccharide, sigMAVisc™, or Eleview™ (each, n = 6) as an SFC. The effects of these agents on wound healing were examined. We evaluated the efficacy and safety of endoscopic surgery after EndoClot®SIS polysaccharide injection.
Results
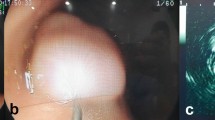
EndoClot®SIS polysaccharide produced a longer-lasting elevation with clearer margins than was achieved by sigMAVisc™, Eleview™, or 0.9% NaCl and thereby enabled precise ESD without complications, such as bleeding and perforation. No obvious histopathological damage was observed at the injection site on endoscopy and histology.
Conclusion
Submucosally injected EndoClot®SIS polysaccharide increased the effective separation of the mucosa and submucosa and reduced surgical complications. Hence, EndoClot®SIS polysaccharide injection is a safe and effective submucosal injection material.






Similar content being viewed by others
References
Ono H, Kondo H, Gotoda T, et al. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225–229.
Jung HY. Extended approach of EMR/ESD in stomach cancer: CON. J Korean Gastric Cancer Assoc. 2008;8:5–8.
Oka S, Tanaka S, Kaneko I, et al. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877–883.
Sumiyama K, Gostout CJ, Rajan E, et al. Submucosal endoscopy with mucosal flap safety valve. Gastrointest Endosc. 2007;65:688–694.
Uraoka T, Saito Y, Yamamoto K, et al. Submucosal injection solution for gastrointestinal tract endoscopic mucosal resection and endoscopic submucosal dissection. Drug Des Devel Ther. 2009;2:131–138.
Fujishiro M, Yahagi N, Kashimura K, et al. Tissue damage of different submucosal injection solutions for EMR. Gastrointest Endosc. 2005;62:933–942.
Thorlacius H, Uedo N, Toth E. Implementation of endoscopic submucosal dissection for early colorectal neoplasms in Sweden. Gastroenterol Res Pract. 2013;2013:758202.
Yamamoto H, Yube T, Isoda N, et al. A novel method of endoscopic mucosal resection using sodium hyaluronate. Gastrointest Endosc. 1999;50:251–256.
Yamamoto H, Koiwai H, Yube T, et al. A successful single-step endoscopic resection of a 40 millimeter flat-elevated tumor in the rectum: endoscopic mucosal resection using sodium hyaluronate. Gastrointest Endosc. 1999;50:701–704.
Hirao M, Masuda K, Asanuma T, et al. Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline–epinephrine. Gastrointest Endosc. 1988;34:264–269.
Makuuchi H, Mitomi T, Machimura T, et al. Endoscopic mucosal resection for early esophageal cancer by EMR-tube method. Stomach Intest. 1988;28:153–159.
Ishizuka T, Hayashi T, Ishihara M, et al. Submucosal injection, for endoscopic mucosal resection, of photocrosslinkable chitosan hydrogel in DMEM/F12 medium. Endoscopy. 2007;39:428–433.
Hayashi T, Matsuyama T, Hanada K, et al. Usefulness of photocrosslinkable chitosan for endoscopic cancer treatment in alimentary tract. J Biomed Mater Res B Appl Biomater. 2004;71:367–372.
Spadaccini M, Hassan C, Maselli R, et al. Efficacy and safety of SIC-8000 (Eleview(R)) for submucosal injection for endoscopic mucosal resection and endoscopic submucosal dissection in an in vivo porcine model. Dig Liver Dis. 2018;50:260–266.
Conio M, Rajan E, Sorbi D, et al. Comparative performance in the porcine esophagus of different solutions used for submucosal injection. Gastrointest Endosc. 2002;56:513–516.
Fujishiro M, Yahagi N, Kashimura K, et al. Comparison of various submucosal injection solutions for maintaining mucosal elevation during endoscopic mucosal resection. Endoscopy. 2004;36:579–583.
Yamamoto H, Yahagi N, Oyama T, et al. Usefulness and safety of 0.4% sodium hyaluronate solution as a submucosal fluid “cushion” in endoscopic resection for gastric neoplasms: a prospective multicenter trial. Gastrointest Endosc. 2008;67:830–839.
Hirasaki S, Kozu T, Yamamoto H, et al. Usefulness and safety of 0.4% sodium hyaluronate solution as a submucosal fluid “cushion” for endoscopic resection of colorectal mucosal neoplasms: a prospective multi-center open-label trial. BMC Gastroenterol. 2009;9:1.
Matsui Y, Inomata M, Izumi K, et al. Hyaluronic acid stimulates tumor-cell proliferation at wound sites. Gastrointest Endosc. 2004;60:539–543.
Ispas-Szabo P, De Koninck P, Calinescu C, et al. Carboxymethyl starch excipients for drug chronodelivery. AAPS PharmSciTech. 2017;18:1673–1682.
Liu W, Zhao M, Liu W, et al. A feasibility study of a thermally sensitive elastin-like polypeptide for submucosal injection application in endoscopic resection in 3 animal models. Gastrointest Endosc. 2015;82:944–952.
Yu L, Xu W, Shen W, et al. Poly(lactic acid-co-glycolic acid)–poly(ethylene glycol)–poly(lactic acid-co-glycolic acid) thermogel as a novel submucosal cushion for endoscopic submucosal dissection. Acta Biomater. 2014;10:1251–1258.
Leonida M, Ispas-Szabo P, Mateescu MA. Self-stabilized chitosan and its complexes with carboxymethyl starch as excipients in drug delivery. Bioact Mater. 2018;3:334–340.
Tada M, Shimada M, Murakami F. Development of strip-off biopsy. Gastroenterol Endosc. 1984;26:833–839.
Hyun JJ, Chun HR, Chun HJ, et al. Comparison of the characteristics of submucosal injection solutions used in endoscopic mucosal resection. Scand J Gastroenterol. 2006;41:488–492.
Giday SA, Magno P, Buscaglia JM, et al. Is blood the ideal submucosal cushioning agent? A comparative study in a porcine model. Endoscopy. 2006;38:1230–1234.
Repici A, Wallace M, Sharma P, et al. A novel submucosal injection solution for endoscopic resection of large colorectal lesions: a randomized, double-blind trial. Gastrointest Endosc. 2018;88:527.e5–535.e5.
Kang KJ, Min BH, Lee JH, et al. Alginate hydrogel as a potential alternative to hyaluronic acid as submucosal injection material. Dig Dis Sci. 2013;58:1491–1496.
Gostout CJ. Ode to the submucosal fluid cushion. Endoscopy. 2004;36:638–639.
Akagi T, Yasuda K, Tajima M, et al. Sodium alginate as an ideal submucosal injection material for endoscopic submucosal resection: preliminary experimental and clinical study. Gastrointest Endosc. 2011;74:1026–1032.
Sumiyoshi T, Fujii T, Sumiyoshi Y, et al. Injected substances to the submucosa in endoscopic mucosal resection-glycerin solution versus normal saline solution. Gastrointest Endosc. 2002;55:AB110.
Liu W, Wang M, Zhao L, et al. Thermo-sensitive isopentane aerification for mucosal lift during endoscopic resection in animal models (with video). Gastrointest Endosc. 2017;86:1168.e3–1175.e3.
Kumano I, Ishihara M, Nakamura S, et al. Endoscopic submucosal dissection for pig esophagus by using photocrosslinkable chitosan hydrogel as submucosal fluid cushion. Gastrointest Endosc. 2012;75:841–848.
Yamasaki M, Kume K, Yoshikawa I, et al. A novel method of endoscopic submucosal dissection with blunt abrasion by submucosal injection of sodium carboxymethylcellulose: an animal preliminary study. Gastrointest Endosc. 2006;64:958–965.
Acknowledgments
The authors are grateful to Suzhou AnDeJia Biotechnology Co., Ltd. for providing injection materials.
Funding
This study was supported by grants from the Jiangsu Province Young Medical Talents Program (No. 2016-2020) and the Health Bureau of Suzhou (Nos. SYZ201632 and SS201641).
Author information
Authors and Affiliations
Contributions
Ming-Sen Dai and Ke-Wei Hu designed the study; Guo-Jian Yin, Wei Wu, Duan-Min Hu performed the experiments; and Ming-Sen Dai analyzed the data and, and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dai, MS., Hu, KW., Wu, W. et al. EndoClot®SIS Polysaccharide Injection as a Submucosal Fluid Cushion for Endoscopic Mucosal Therapies: Results of Ex Vivo and In Vivo Studies. Dig Dis Sci 64, 2955–2964 (2019). https://doi.org/10.1007/s10620-019-05686-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-019-05686-4