Abstract
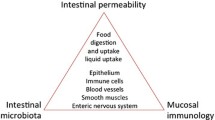
For decades, the pathogenesis of a variety of human diseases has been attributed to increased intestinal paracellular permeability even though scientific evidence supporting this hypothesis has been tenuous. Nevertheless, during the past decade, there have been a growing number of publications focused on human genetics, the gut microbiome, and proteomics, suggesting that loss of mucosal barrier function, particularly in the gastrointestinal tract, may substantially affect antigen trafficking, ultimately causing chronic inflammation, including autoimmunity, in genetically predisposed individuals. The gut mucosa works as a semipermeable barrier in that it permits nutrient absorption and also regulates immune surveillance while retaining potentially harmful microbes and environmental antigens within the intestinal lumen. Celiac disease (CD), a systemic, immune-mediated disorder triggered by gluten in genetically susceptible individuals, is associated with altered gut permeability. Pre-clinical and clinical studies have shown that gliadin, a prolamine component of gluten that is implicated in CD pathogenesis, is capable to disassembling intercellular junctional proteins by upregulating the zonulin pathway, which can be inhibited by the zonulin antagonist larazotide acetate. In this review, we will focus on CD as a paradigm of chronic inflammatory diseases in order to outline the contribution of gut paracellular permeability toward disease pathogenesis; moreover, we will summarize current evidence derived from available clinical trials of larazotide acetate in CD.



Similar content being viewed by others
References
Husby S, Koletzko S, Korponay-Szabo IR, et al. European society for pediatric gastroenterology, hepatology, and nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136–160.
Catassi C, Kryszak D, Bhatti B, et al. Natural history of celiac disease autoimmunity in a USA cohort followed since 1974. Ann Med. 2010;42:530–538.
Lionetti E, Castellaneta S, Francavilla R, et al. Introduction of gluten, HLA status, and the risk of celiac disease in children. N Engl J Med. 2014;371:1295–1303.
Fasano A. Celiac disease—how to handle a clinical chameleon. N Engl J Med. 2003;348:2568–2570.
Tapsas D, Hollén E, Stenhammar L, et al. The clinical presentation of coeliac disease in 1030 Swedish children: changing features over the past four decades. Dig Liver Dis. 2016;48:16–22.
Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–920.
Händel N, Mothes T, Petroff D, et al. Will the real coeliac disease please stand up? Coeliac disease prevalence in the German LIFE Child Study. J Pediatr Gastroenterol Nutr. 2018;67:494–500.
Singh P, Arora S, Singh A, et al. Prevalence of celiac disease in Asia: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31:1095–1101.
Parra-Medina R, Molano-Gonzalez N, Rojas-Villarraga A, et al. Prevalence of celiac disease in latin america: a systematic review and meta-regression. PLoS ONE. 2015;10:e0124040.
Ege MJ. The hygiene hypothesis in the age of the microbiome. Ann Am Thorac Soc. 2017;14:S348–S353.
De Re V, Magris R, Cannizzaro R. New insights into the pathogenesis of celiac disease. Front Med. 2017;31:137.
Vancamelbeke M, Vermeire S. The intestinal barrier: a fundamental role in health and disease. Expert Rev Gastroenterol Hepatol. 2017;11:821–834.
Okumura R, Takeda K. Maintenance of intestinal homeostasis by mucosal barriers. Inflamm Regen. 2018;38:5.
Rodriguez-Pineiro AM, Bergstrom JH, et al. Studies of mucus in mouse stomach, small intestine, and colon. Gastrointestinal mucus proteome reveals Muc2 and Muc5ac accompanied by a set of core proteins. Am J Physiol Gastrointest Liver Physiol. 2013;305:G348–G356.
Moran AP, Gupta A, Joshi L. Sweet-talk: role of host glycosylation in bacterial pathogenesis of the gastrointestinal tract. Gut. 2011;60:1412–1425.
Groschwitz KR, Hogan SP. Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol. 2009;124:3–20.
Duffey ME, Hainau B, Ho S, et al. Regulation of epithelial tight junction permeability by cyclic AMP. Nature. 1981;294:451–453.
Itoh M, Nagafuchi A, Yonemura S, et al. The 220-kD protein colocalizing with cadherins in non-epithelial cells is identical to ZO-1, a tight junction-associated protein in epithelial cells: cDNA cloning and immunoelectron microscopy. J Cell Biol. 1993;121:491–502.
Furuse M, Hirase T, Itoh M, et al. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788.
Furuse M, Fujita K, Hiiragi T, et al. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occluding. J Cell Biol. 1998;141:1539–1550.
Martin-Padura I, Lostaglio S, Schneemann M, et al. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 1998;142:117–127.
Ikenouchi J, Furuse M, Furuse K, et al. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol. 2005;171:939–945.
Higashi T, Tokuda S, Kitajiri S, et al. Analysis of the ‘angulin’ proteins LSR, ILDR1 and ILDR2–tricellulin recruitment, epithelial barrier function and implication in deafness pathogenesis. J Cell Sci. 2013;126:966–977.
Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451–1455.
Mazzilli MC, Ferrante P, Mariani P, et al. A study of Italian pediatric celiac disease patients confirms that the primary HLA association is to the DQ(alpha 1*0501, beta 1*0201) heterodimer. Hum Immunol. 1992;33:133–139.
Lundin KE, Wijmenga C. Coeliac disease and autoimmune disease-genetic overlap and screening. Nat Rev Gastroenterol Hepatol. 2015;12:507–515.
Dieli-Crimi R, Cenit MC, Nunez C. The genetics of celiac disease: a comprehensive review of clinical implications. J Autoimmun. 2015;64:26–41.
Williamson IA, Arnold JW, Samsa LA, et al. A high-throughput organoid microinjection platform to study gastrointestinal microbiota and luminal physiology. Cell Mol Gastroenterol Hepatol. 2018;6:301–319.
Bach JF. The hygiene hypothesis in autoimmunity: the role of pathogens and commensals. Nat Rev Immunol. 2018;18:105–120.
Rao DA. T cells that help B cells in chronically inflamed tissues. Front Immunol. 2018;9:1924.
Kim SM, Mayassi T, Jabri B. Innate immunity: actuating the gears of celiac disease pathogenesis. Best Pract Res Clin Gastroenterol. 2015;29:425–435.
Lammers KM, Lu R, Brownley J, et al. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR32. Gastroenterology. 2008;135:194–204 e193.
Sellitto M, Bai G, Serena G, et al. Proof of concept of microbiome-metabolome analysis and delayed gluten exposure on celiac disease autoimmunity in genetically at-risk infants. PLoS ONE. 2012;7:e33387.
Tripathi A, Lammers KM, Goldblum S, et al. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc Natl Acad Sci USA. 2009;106:16799–16804.
Asleh R, Marsh S, Shilkrut M, et al. Genetically determined heterogeneity in hemoglobin scavenging and susceptibility to diabetic cardiovascular disease. Circ Res. 2003;92:1193–1200.
Wicher KB, Fries E. Prohaptoglobin is proteolytically cleaved in the endoplasmic reticulum by the complement C1r-like protein. Proc Natl Acad Sci. 2004;101:14390–14395.
Drago S, El Asmar R, Di Pierro M, et al. Gliadin, zonulin and gut permeability: effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand J Gastroenterol. 2006;41:408–419.
Kurosky A, Barnett DR, Lee TH, et al. Covalent structure of human haptoglobin: a serine protease homolog. Proc Natl Acad Sci USA. 1980;77:3388–3392.
Wicher KB, Fries E. Haptoglobin, a hemoglobin-binding plasma protein, is present in bony fish and mammals but not in frog and chicken. Proc Natl Acad Sci. 2006;103:4168–4173.
Nielsen MJ, Petersen SV, Jacobsen C, et al. A unique loop extension in the serine protease domain of haptoglobin is essential for CD163 recognition of the haptoglobin-hemoglobin complex. J Biol Chem. 2007;282:1072–1079.
Polticelli F, Bocedi A, Minervini G, et al. Human haptoglobin structure and function—a molecular modelling study. FEBS J. 2008;275:5648–5656.
El Asmar R, Panigrahi P, Bamford P, et al. Host-dependent activation of the zonulin system is involved in the impairment of the gut barrier function following bacterial colonization. Gastroenterology. 2002;123:1607–1615.
Thomas KE, Fasano A, Vogel SN. Gliadin stimulation of murine macrophage inflammatory gene expression and intestinal permeability are MyD88-dependent: role of the innate immune response in Celiac disease. J Immunol. 2006;176:2512–2521.
Scheffler L, Crane A, Heyne H, et al. Widely used commercial ELISA does not detect precursor of haptoglobin 2, but recognizes properdin as a potential second member of the zonulin family. Front Endocrinol. 2018;5:22.
Kouser L, Abdul-Aziz M, Nayak A, et al. Properdin and factor H: opposing players on the alternative complement pathway “see–saw”. Front Immunol. 2013;4:93.
Rittirsch D, Flierl MA, Nadeau BA, et al. Zonulin as prehaptoglobin2 regulates lung permeability and activates the complement system. Am J Physiol. 2013;304:72.
Shirey KA, Lai W, Patel MC, et al. Novel strategies for targeting innate immune responses to influenza. Mucosal Immunol. 2016;9:1173–1182.
Ahout IM, Brand KH, Zomer A, et al. Prospective observational study in two Dutch hospitals to assess the performance of inflammatory plasma markers to determine disease severity of viral respiratory tract infections in children. BMJ Open. 2017;7:e014596.
Ajamian M, Steer D, Rosella G, Gibson PR. Serum zonulin as a marker of intestinal mucosal barrier function: may not be what it seems. PLoS ONE. 2019;14:e0210728.
Hollande F, Blanc EM, Bali JP. HGF regulates tight junctions in new nontumorigenic gastric epithelial cell line. Am J Physiol Gastrointest Liver Physiol. 2001;280:G910–G921.
van der Merwe JQ, Hollenberg MD, MacNaughton WK. EGF receptor transactivation and MAP kinase mediate proteinase-activated receptor-2-induced chloride secretion in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2008;294:G441–G451.
Cenac N, Chin AC, Garcia-Villar R, et al. PAR2 activation alters colonic paracellular permeability in mice via IFN-gamma-dependent and -independent pathways. J Physiol. 2004;558:913–925.
Brown GR, Lindberg G, Meddings J, et al. Tumor necrosis factor inhibitor ameliorates murine intestinal graft-versus-host disease. Gastroenterology. 1999;116:593–601.
Clemente MG, De Virgiliis S, Kang JS, et al. Early effects of gliadin on enterocyte intracellular signaling involved in intestinal barrier function. Gut. 2003;52:218–223.
Jelinkova L, Tuckova L, Cinova J, et al. Gliadin stimulates human monocytes to production of IL-8 and TNF-alpha through a mechanism involving NF-kappaB. FEBS Lett. 2004;571:81–85.
Silano M, Vincentini O, De Vincenzi M. Toxic, immunostimulatory and antagonist gluten peptides in celiac disease. Curr Med Chem. 2009;16:1489–1498.
Lammers KM, Khandelwal S, Chaudhry F, et al. Identification of a novel immunomodulatory gliadin peptide that causes interleukin-8 release in a chemokine receptor CXCR57-dependent manner only in patients with coeliac disease. Immunology. 2011;132:432–440.
Sapone A, de Magistris L, Pietzak M, et al. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes. 2006;55:1443–1449.
Barone MV, Gimigliano A, Castoria G, et al. Growth factor-like activity of gliadin, an alimentary protein: implications for coeliac disease. Gut. 2007;56:480–488.
Wang W, Uzzau S, Goldblum SE, et al. Human zonulin, a potential modulator of intestinal tight junctions. J Cell Sci. 2000;113:4435–4440.
Monsuur AJ, de Bakker PI, Alizadeh BZ, et al. Myosin IXB variant increases the risk of celiac disease and points toward a primary intestinal barrier defect. Nat Genet. 2005;37:1341–1344.
Wapenaar MC, Monsuur AJ, van Bodegraven AA, et al. Associations with tight junction genes PARD3 and MAGI2 in Dutch patients point to a common barrier defect for coeliac disease and ulcerative colitis. Gut. 2008;57:463–467.
Schumann M, Richter JF, Wedell I, et al. Mechanisms of epithelial translocation of the alpha(2)-gliadin-33mer in coeliac sprue. Gut. 2008;57:747–754.
Matysiak-Budnik T, Moura IC, Arcos-Fajardo M, et al. Secretory IgA mediates retrotranscytosis of intact gliadin peptides via the transferrin receptor in celiac disease. J Exp Med. 2008;205:143–154.
Cario E, Rosenberg IM, Brandwein SL, et al. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol. 2000;164:966–972.
Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology. 2004;127:224–238.
Marafini I, Monteleone I, Di Fusco D, et al. TNF-alpha producing innate lymphoid cells (ILCs) are increased in active celiac disease and contribute to promote intestinal atrophy in mice. PLoS ONE. 2015;10:e0126291.
Noth R, Stuber E, Hasler R, et al. Anti-TNF-alpha antibodies improve intestinal barrier function in Crohn’s disease. J Crohns Colitis. 2012;6:464–469.
Barone MV, Troncone R, Auricchio S. Gliadin peptides as triggers of the proliferative and stress/innate immune response of the celiac small intestinal mucosa. Int J Mol Sci. 2014;15:20518–20537.
Reig-Otero Y, Mañes J, Manyes L. Amylase-trypsin inhibitors in wheat and other cereals as potential activators of the effects of nonceliac gluten sensitivity. J Med Food. 2018;21:207–214.
Cinova J, Palova-Jelinkova L, Smythies LE, et al. Gliadin peptides activate blood monocytes from patients with celiac disease. J Clin Immunol. 2007;27:201–209.
Stamnaes J, Sollid LM. Celiac disease: Autoimmunity in response to food antigen. Semin Immunol. 2015;27:343–352.
van de Wal Y, Kooy YMC, van-Veelen P, et al. Selective deamidation by tissue transglutaminase strongly enhances gliadin-specific T cell reactivity. J Immunol. 1998;161:1585–1588.
Meresse B, Korneychuk N, Malamut G, et al. Interleukin-15, a master piece in the immunological jigsaw of celiac disease. Dig Dis. 2015;33:122–130.
Shibahara T, Wilcox JN, Couse T, et al. Characterization of epithelial chemoattractants for human intestinal intraepithelial lymphocytes. Gastroenterology. 2001;120:60–70.
Salvati VM, Troncone R, Bajaj-Elliott M, et al. Keratinocyte growth factor and coeliac disease. Gut. 2001;49:176–181.
Tang F, Chen Z, Ciszewski C, et al. Cytosolic PLA2 is required for CTL-mediated immunopathology of celiac disease via NKG2D and IL-15. J Exp Med. 2009;206:707–719.
Senger S, Sapone A, Fiorentino MR, et al. Celiac disease histopathology recapitulates hedgehog downregulation, consistent with wound healing processes activation. PLoS ONE. 2015;10:e0144634.
See JA, Kaukinen K, Makharia GK, et al. Practical insights into gluten-free diets. Nat Rev Gastroenterol Hepatol. 2015;12:580–591.
Valitutti F, Trovato CM, Montuori M, et al. Pediatric celiac disease: follow-up in the spotlight. Adv Nutr. 2017;8:356–361.
Hall NJ, Rubin G, Charnock A. Systematic review: adherence to a gluten-free diet in adult patients with celiac disease. Aliment Pharmacol Ther. 2009;15:315–330.
Branchi F, Tomba C, Ferretti F, et al. Celiac disease and drug-based therapies: inquiry into patients demands. Digestion. 2016;93:160–166.
Norsa L, Tomba C, Agostoni C, et al. Gluten-free diet or alternative therapy: a survey on what parents of celiac children want. Int J Food Sci Nutr. 2015;66:590–594.
Sturgeon C, Fasano A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers. 2016;4:e1251384.
Gopalakrishnan S, Durai M, Kitchens K, et al. Larazotide acetate regulates epithelial tight junctions in vitro and in vivo. Peptides. 2012;35:86–94.
Paterson BM, Lammers KM, Arrieta MC, et al. The safety, tolerance, pharmacokinetic and pharmacodynamic effects of single doses of AT-1001 in coeliac disease subjects: a proof of concept study. Aliment Pharmacol Ther. 2007;26:757–766.
Leffler DA, Kelly CP, Abdallah HZ, et al. A randomized, double-blind study of larazotide acetate to prevent the activation of celiac disease during gluten challenge. Am J Gastroenterol. 2012;107:1554–1562.
Kelly CP, Green PH, Murray JA, et al. Larazotide acetate in patients with coeliac disease undergoing a gluten challenge: a randomised placebo-controlled study. Aliment Pharmacol Ther. 2013;37:252–262.
Leffler D, Kelly C, Green P, et al. Larazotide acetate for persistent symptoms of celiac disease despite a gluten-free diet: a randomized controlled trial. Gastroenterology. 2015;148:1311–1319.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Fasano is co-founder and stock holder of Alba Therapeutics, a company developing treatments complementary to the gluten-free diet by exploiting gut permeability; Dr. Valitutti has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Valitutti, F., Fasano, A. Breaking Down Barriers: How Understanding Celiac Disease Pathogenesis Informed the Development of Novel Treatments. Dig Dis Sci 64, 1748–1758 (2019). https://doi.org/10.1007/s10620-019-05646-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-019-05646-y