Abstract
Background
Although ClC-2 channels are important in colonic Cl− secretion, it is unclear about their roles in small intestinal anion secretion. Therefore, we sought to examine whether ClC-2 channels play important roles in anion secretion, particularly duodenal bicarbonate secretion (DBS).
Methods
Duodenal mucosae from mice were stripped of seromuscular layers and mounted in Ussing chambers. Both duodenal short-circuit current (Isc) and HCO3− secretion in vitro were simultaneously recorded. DBS in vivo was measured by a CO2-sensitive electrode.
Results
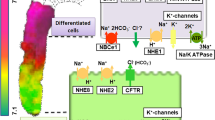
Lubiprostone, a selective ClC-2 activator, concentration-dependently increased both duodenal Isc and DBS only when applied basolaterally, but not when applied apically. Removal of extracellular Cl− abolished lubiprostone-induced duodenal Isc, but did not alter HCO3− secretion even in the presence of DIDS, a Cl−/HCO3− exchanger inhibitor. However, further addition of glibenclamide, a CFTR channel blocker, abolished lubiprostone-evoked HCO3− secretion. Moreover, lubiprostone-induced HCO3− secretion was impaired in CFTR−/− mice compared to wild-type littermates. Luminal perfusion of duodenal lumen with lubiprostone did not alter basal DBS in vivo, but lubiprostone (i.p.) was able to induce DBS, which was also significantly inhibited by Cd2+, a ClC-2 channel blocker. [Ca2+]cyt level, Ca2+-activated K+ channel- and cAMP-mediated duodenal Isc, and HCO3− secretion were unchanged by lubiprostone.
Conclusions
We have provided the first evidence for the novel functional role of basolateral ClC-2 channels in the regulation of duodenal anion secretion.






Similar content being viewed by others
Abbreviations
- CFTR:
-
Cystic fibrosis transmembrane conductance regulator
- DBS:
-
Duodenal bicarbonate secretion
- AE:
-
Anion exchangers
- NBC:
-
Na+/HCO3− cotransporters
- TER:
-
Transepithelial resistance
References
Montrose MH, Keely S, Barrett K. Electrolyte secretion and absorption: small intestine and colon. In: Yamada T, ed. Textbook of Gastroenterology, vol. one. 4th ed. Philadephia, PA: Lippincott Williams & Wilkins; 2003:308–340.
Barrett KE. Integrated regulation of intestinal epithelial transport: intercellular and intracellular pathways. Am J Physiol. 1997;272:C1069–C1076.
Isenberg JI, Selling JA, Hogan DL, Koss MA. Impaired proximal duodenal mucosal bicarbonate secretion in patients with duodenal ulcer. N Engl J Med. 1987;316:374–379.
Allen A, Flemstrom G. Gastroduodenal mucus bicarbonate barrier: protection against acid and pepsin. Am J Physiol Cell Physiol. 2005;288:C1–C19.
Flemstrom G, Isenberg JI. Gastroduodenal mucosal alkaline secretion and mucosal protection. News Physiol Sci. 2001;16:23–28.
Gyomorey K, Yeger H, Ackerley C, Garami E, Bear CE. Expression of the chloride channel ClC-2 in the murine small intestine epithelium. Am J Physiol Cell Physiol. 2000;279:C1787–C1794.
Pena-Munzenmayer G, Catalan M, Cornejo I, et al. Basolateral localization of native ClC-2 chloride channels in absorptive intestinal epithelial cells and basolateral sorting encoded by a CBS-2 domain di-leucine motif. J Cell Sci. 2005;118:4243–4252.
Mohammad-Panah R, Gyomorey K, Rommens J, et al. ClC-2 contributes to native chloride secretion by a human intestinal cell line, Caco-2. J Biol Chem. 2001;276:8306–8313.
Cuppoletti J, Malinowska DH, Tewari KP, et al. SPI-0211 activates T84 cell chloride transport and recombinant human ClC-2 chloride currents. Am J Physiol Cell Physiol. 2004;287:C1173–C1183.
Flores CA. ClC-2 and intestinal chloride secretion. Am J Physiol Gastrointest Liver Physiol. 2016;311:G775.
Lipecka J, Bali M, Thomas A, Fanen P, Edelman A, Fritsch J. Distribution of ClC-2 chloride channel in rat and human epithelial tissues. Am J Physiol Cell Physiol. 2002;282:C805–C816.
Catalan M, Cornejo I, Figueroa CD, Niemeyer MI, Sepulveda FV, Cid LP. ClC-2 in guinea pig colon: mRNA, immunolabeling, and functional evidence for surface epithelium localization. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1004–G1013.
Jakab RL, Collaco AM, Ameen NA. Lubiprostone targets prostanoid signaling and promotes ion transporter trafficking, mucus exocytosis, and contractility. Dig Dis Sci. 2012;57:2826–2845. https://doi.org/10.1007/s10620-012-2352-8
Bijvelds MJC, Bot AGM, Escher JC, de Jonge HR. Activation of intestinal Cl− secretion by lubiprostone requires the cystic fibrosis transmembrane conductance regulator. Gastroenterology. 2009;137:976–985.
Ao M, Venkatasubramanian J, Boonkaewwan C, et al. Lubiprostone activates Cl− secretion via cAMP signaling and increases membrane CFTR in the human colon carcinoma cell line, T84. Dig Dis Sci. 2011;56:339–351. https://doi.org/10.1007/s10620-010-1495-8
Hogan DL, Crombie DL, Isenberg JI, Svendsen P, Schaffalitzky DMO, Ainsworth MA. CFTR mediates cAMP- and Ca2+-activated duodenal epithelial HCO3 − secretion. Am J Physiol. 1997;272:G872–G878.
Hogan DL, Crombie DL, Isenberg JI, Svendsen P, Schaffalitzky DMO, Ainsworth MA. Acid-stimulated duodenal bicarbonate secretion involves a CFTR-mediated transport pathway in mice. Gastroenterology. 1997;113:533–541.
Snouwaert JN, Brigman KK, Latour AM, et al. An animal model for cystic fibrosis made by gene targeting. Science. 1992;257:1083–1088.
Clarke LL, Gawenis LR, Franklin CL, Harline MC. Increased survival of CFTR knockout mice with an oral osmotic laxative. Lab Anim Sci. 1996;46:612–618.
Dong H, Sellers ZM, Smith A, Chow JY, Barrett KE. Na(+)/Ca(2+) exchange regulates Ca(2+)-dependent duodenal mucosal ion transport and HCO(3)(−) secretion in mice. Am J Physiol Gastrointest Liver Physiol. 2005;288:G457–G465.
Dong H, Smith A, Hovaida M, Chow JY. Role of Ca2+-activated K+ channels in duodenal mucosal ion transport and bicarbonate secretion. Am J Physiol Gastrointest Liver Physiol. 2006;291:G1120–G1128.
Pang G, Buret A, O'Loughlin E, Smith A, Batey R, Clancy R. Immunologic, functional, and morphological characterization of three new human small intestinal epithelial cell lines. Gastroenterology. 1996;111:8–18.
Buresi MC, Schleihauf E, Vergnolle N, et al. Protease-activated receptor-1 stimulates Ca(2+)-dependent Cl(−) secretion in human intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2001;281:G323–G332.
Smith AJ, Chappell AE, Buret AG, Barrett KE, Dong H. 5-Hydroxytryptamine contributes significantly to a reflex pathway by which the duodenal mucosa protects itself from gastric acid injury. FASEB J. 2006;20:2486–2495.
Cuppoletti J, Chakrabarti J, Tewari KP, Malinowska DH. Differentiation between human ClC-2 and CFTR Cl− channels with pharmacological agents. Am J Physiol Cell Physiol. 2014;307:C479–C492.
Bao HF, Liu L, Self J, Duke BJ, Ueno R, Eaton DC. A synthetic prostone activates apical chloride channels in A6 epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2008;295:G234–G251.
Tuo B, Riederer B, Wang Z, Colledge WH, Soleimani M, Seidler U. Involvement of the anion exchanger SLC26A6 in prostaglandin E2-but not forskolin-stimulated duodenal HCO3 − secretion. Gastroenterology. 2006;130:349–358.
Spiegel S, Phillipper M, Rossmann H, Riederer B, Gregor M, Seidler U. Independence of apical Cl−/HCO3 − exchange and anion conductance in duodenal HCO3 − secretion. Am J Physiol Gastrointest Liver Physiol. 2003;285:G887–G897.
Reddy MM, Quinton PM. Effect of anion transport blockers on CFTR in the human sweat duct. J Membr Biol. 2002;189:15–25.
Ito Y, Son M, Sato S, et al. ATP release triggered by activation of the Ca2+-activated K+ channel in human airway Calu-3 cells. Am J Respir Cell Mol Biol. 2004;30:388–395.
Abdullaev IF, Rudkouskaya A, Schools GP, Kimelberg HK, Mongin AA. Pharmacological comparison of swelling-activated excitatory amino acid release and Cl− currents in cultured rat astrocytes. J Physiol. 2006;572:677–689.
Devor DC, Singh AK, Gerlach AC, Frizzell RA, Bridges RJ. Inhibition of intestinal Cl− secretion by clotrimazole: direct effect on basolateral membrane K+ channels. Am J Physiol. 1997;273:C531–C540.
Devor DC, Singh AK, Bridges RJ, Frizzell RA. Modulation of Cl− secretion by benzimidazolones. II. Coordinate regulation of apical GCl and basolateral GK. Am J Physiol. 1996;271:L785–L795.
Devor DC, Singh AK, Frizzell RA, Bridges RJ. Modulation of Cl− secretion by benzimidazolones. I. Direct activation of a Ca(2+)-dependent K+ channel. Am J Physiol. 1996;271:L775–L784.
Wulff H, Miller MJ, Hansel W, Grissmer S, Cahalan MD, Chandy KG. Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: a potential immunosuppressant. Proc Natl Acad Sci USA. 2000;97:8151–8156.
Zdebik AA, Cuffe J, Bertog M. Additional disruption of the ClC-2 Cl(−) channel does not exacerbate the cystic fibrosis phenotype of cystic fibrosis transmembrane conductance regulator mouse models. J Biol Chem. 2004;279:22276–22283.
Catalan M, Niemeyer MI, Cid LP, Sepulveda FV. Basolateral ClC-2 chloride channels in surface colon epithelium: regulation by a direct effect of intracellular chloride. Gastroenterology. 2004;126:1104–1114.
Mizumori M, Akiba Y, Kaunitz JD. Lubiprostone stimulates duodenal bicarbonate secretion in rats. Dig Dis Sci. 2009;54:2063–2069. https://doi.org/10.1007/s10620-009-0907-0
Cuppoletti J, Tewari KP, Chakrabarti J, Malinowska DH. Identification of the fatty acid activation site on human ClC-2. Am J Physiol Cell Physiol. 2017;312:C707–C723.
Thiemann A, Grunder S, Pusch M, Jentsch TJ. A chloride channel widely expressed in epithelial and non-epithelial cells. Nature. 1992;356:57–60.
Bali M, Lipecka J, Edelman A, Fritsch J. Regulation of ClC-2 chloride channels in T84 cells by TGF-alpha. Am J Physiol Cell Physiol. 2001;280:C1588–C1598.
Murray CB, Chu S, Zeitlin PL. Gestational and tissue-specific regulation of C1C-2 chloride channel expression. Am J Physiol. 1996;271:L829–L837.
Kajita H, Omori K, Matsuda H. The chloride channel ClC-2 contributes to the inwardly rectifying Cl− conductance in cultured porcine choroid plexus epithelial cells. J Physiol. 2000;523:313–324.
Ueno R, Osama H, Habe T, Engelke K. Oral SPI-0211 increases intestinal fluid secretion and chloride concentration without altering serum electrolyte levels (abstract). Gastroenterology. 2004;126:A-298.
Johansen JF, Gargano M, Holland P, Patchen M. Phase III, efficacy and safety of RU-0211 a novel chloride channel activator, for the treatment of constipation (Abstract). Gastroenterology. 2003;124:A48.
Kim SW, Parekh D, Townsend CJ, Thompson JC. Effects of aging on duodenal bicarbonate secretion. Ann Surg. 1990;212:332–337, 337–338.
Valle JD, Chey W, Scheiman J. Acid peptic disorders. In: Yamada T, ed. Textbook of Gastroenterology, vol. one. 4th ed. Philadephia, PA: Lippincott Williams & Wilkins; 2003.
Moeser AJ, Nighot PK, Engelke KJ, Ueno R, Blikslager AT. Recovery of mucosal barrier function in ischemic porcine ileum and colon is stimulated by a novel agonist of the ClC-2 chloride channel, lubiprostone. Am J Physiol-Gastr L. 2007;292:G647–G656.
Acknowledgments
These studies were supported by research grants from the National Key Research and Development Program of China (No. 2016YFC1302200 to HD) and the National Natural Science Foundation of China (Nos. 81570477 and 81873544 to HD).
Author information
Authors and Affiliations
Contributions
HD designed and supervised the project, wrote and finalized the manuscript. CD and JL conducted most experiments and data analysis. HXW conducted some experiments. XZ finalized the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Du, C., Liu, J., Wan, H. et al. Functional Role of Basolateral ClC-2 Channels in the Regulation of Duodenal Anion Secretion in Mice. Dig Dis Sci 64, 2527–2537 (2019). https://doi.org/10.1007/s10620-019-05578-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-019-05578-7