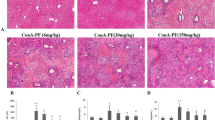
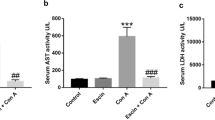
Abstract
Background and Aims
Concanavalin A is known to activate T cells and to cause liver injury and hepatitis, mediated in part by secretion of TNFα from macrophages. Poly(ADP-ribose) polymerase-1 (PARP-1) inhibitors have been shown to prevent tissue damage in various animal models of inflammation. The objectives of this study were to evaluate the efficacy and mechanism of the PARP-1 inhibitor 3-aminobenzamide (3-AB) in preventing concanavalin A-induced liver damage.
Methods
We tested the in vivo effects of 3-AB on concanavalin A-treated mice, its effects on lipopolysaccharide (LPS)-stimulated macrophages in culture, and its ability to act as a scavenger in in vitro assays.
Results
3-AB markedly reduced inflammation, oxidative stress, and liver tissue damage in concanavalin A-treated mice. In LPS-stimulated RAW264.7 macrophages, 3-AB inhibited NFκB transcriptional activity and subsequent expression of TNFα and iNOS and blocked NO production. In vitro, 3-AB acted as a hydrogen peroxide scavenger. The ROS scavenger N-acetylcysteine (NAC) and the ROS formation inhibitor diphenyleneiodonium (DPI) also inhibited TNFα expression in stimulated macrophages, but unlike 3-AB, NAC and DPI were unable to abolish NFκB activity. PARP-1 knockout failed to affect NFκB and TNFα suppression by 3-AB in stimulated macrophages.
Conclusions
Our results suggest that 3-AB has a therapeutic effect on concanavalin A-induced liver injury by inhibiting expression of the key pro-inflammatory cytokine TNFα, via PARP-1-independent NFκB suppression and via an NFκB-independent anti-oxidative mechanism.








Similar content being viewed by others
Abbreviations
- 3-AB:
-
3-Aminobenzamide
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- ConA:
-
Concanavalin A
- DPI:
-
Diphenyleneiodonium
- HRP:
-
Horseradish peroxidase
- IFNγ:
-
Interferon γ
- LPS:
-
Lipopolysaccharide
- MDH:
-
Malate dehydrogenase
- NAC:
-
N-acetylcysteine
- NFκB:
-
Nuclear factor kappa B
- NO:
-
Nitric oxide
- Nox:
-
NADPH oxidase
- PARP-1:
-
Poly(ADP-ribose) polymerase-1
- PF2:
-
Peroxyfluor-2
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- TNFα:
-
Tumor necrosis factor α
References
Tiegs G, Hentschel J, Wendel A. A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J Clin Invest. 1992;90:196–203.
Takeda K, Hayakawa Y, Van Kaer L, Matsuda H, Yagita H, Okumura K. Critical contribution of liver natural killer T cells to a murine model of hepatitis. Proc Natl Acad Sci USA. 2000;97:5498–5503.
Schumann J, Wolf D, Pahl A, et al. Importance of Kupffer cells for T-cell-dependent liver injury in mice. Am J Pathol. 2000;157:1671–1683.
Gantner F, Leist M, Kusters S, Vogt K, Volk HD, Tiegs G. T cell stimulus-induced crosstalk between lymphocytes and liver macrophages results in augmented cytokine release. Exp Cell Res. 1996;229:137–146.
Kusters S, Gantner F, Kunstle G, Tiegs G. Interferon gamma plays a critical role in T cell-dependent liver injury in mice initiated by concanavalin A. Gastroenterology. 1996;111:462–471.
Essani NA, Fisher MA, Jaeschke H. Inhibition of NF-kappa B activation by dimethyl sulfoxide correlates with suppression of TNF-alpha formation, reduced ICAM-1 gene transcription, and protection against endotoxin-induced liver injury. Shock. 1997;7:90–96.
Gill R, Tsung A, Billiar T. Linking oxidative stress to inflammation: toll-like receptors. Free Radic Biol Med. 2010;48:1121–1132.
Roh YS, Seki E. Toll-like receptors in alcoholic liver disease, nonalcoholic steatohepatitis and carcinogenesis. J Gastroenterol Hepatol. 2013;28:38–42.
Xu J, Zhang X, Monestier M, Esmon NL, Esmon CT. Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury. J Immunol. 2011;187:2626–2631.
Gong Q, Zhang H, Li JH, et al. High-mobility group box 1 exacerbates concanavalin A-induced hepatic injury in mice. J Mol Med (Berl). 2010;88:1289–1298.
Woodhouse BC, Dianov GL. Poly(ADP-ribose) polymerase-1: an international molecule of mystery. DNA Repair (Amst). 2008;7:1077–1086.
Liu L, Ke Y, Jiang X, et al. Lipopolysaccharide activates ERK-PARP-1-RelA pathway and promotes nuclear factor-kappaB transcription in murine macrophages. Hum Immunol. 2012;73:439–447.
Huang D, Yang CZ, Yao L, Wang Y, Liao YH, Huang K. Activation and overexpression of PARP-1 in circulating mononuclear cells promote TNF-alpha and IL-6 expression in patients with unstable angina. Arch Med Res. 2008;39:775–784.
Peralta-Leal A, Rodriguez-Vargas JM, Aguilar-Quesada R, et al. PARP inhibitors: new partners in the therapy of cancer and inflammatory diseases. Free Radic Biol Med. 2009;47:13–26.
Mukhopadhyay P, Rajesh M, Cao Z, et al. Poly(ADP-ribose) polymerase-1 is a key mediator of liver inflammation and fibrosis. Hepatology. 2014;59:1998–2009.
Donmez M, Uysal B, Poyrazoglu Y, et al. PARP inhibition prevents acetaminophen-induced liver injury and increases survival rate in rats. Turk J Med Sci. 2015;45:18–26.
Zhang Y, Wang C, Tian Y, et al. Inhibition of poly(ADP-Ribose) polymerase-1 protects chronic alcoholic liver injury. Am J Pathol. 2016;186:3117–3130.
Chen Q, Zhao Y, Cheng Z, Xu Y, Yu C. Establishment of a cell-based assay for examining the expression of tumor necrosis factor alpha (TNF-alpha) gene. Appl Microbiol Biotechnol. 2008;80:357–363.
Conkright MD, Guzman E, Flechner L, Su AI, Hogenesch JB, Montminy M. Genome-wide analysis of CREB target genes reveals a core promoter requirement for cAMP responsiveness. Mol Cell. 2003;11:1101–1108.
Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991;1072:129–157.
Shirin H, Aeed H, Alin A, et al. Inhibition of immune-mediated concanavalin a-induced liver damage by free-radical scavengers. Dig Dis Sci. 2010;55:268–275.
Wills ED. Lipid peroxide formation in microsomes. General considerations. Biochem J. 1969;113:315–324.
Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol. 1995;19:1409–1417.
Fraser I, Liu W, Rebres R, et al. The use of RNA interference to analyze protein phosphatase function in mammalian cells. Methods Mol Biol. 2007;365:261–286.
Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8:2281–2308.
Cong L, Ran FA, Cox D, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823.
Ernst O, Vayttaden SJ, Fraser IDC. Measurement of NF-kappaB activation in TLR-activated macrophages. Methods Mol Biol. 2018;1714:67–78.
Pick E. Cell-free NADPH oxidase activation assays: “in vitro veritas”. Methods Mol Biol. 2014;1124:339–403.
Dickinson BC, Huynh C, Chang CJ. A palette of fluorescent probes with varying emission colors for imaging hydrogen peroxide signaling in living cells. J Am Chem Soc. 2010;132:5906–5915.
Ernst O, Zor T. Linearization of the Bradford protein assay. J Vis Exp. 2010;38:e1918.
Zor T, Selinger Z. Linearization of the Bradford protein assay increases its sensitivity: theoretical and experimental studies. Anal Biochem. 1996;236:302–308.
Hatano M, Sasaki S, Ohata S, et al. Effects of Kupffer cell-depletion on concanavalin A-induced hepatitis. Cell Immunol. 2008;251:25–30.
Koga K, Takaesu G, Yoshida R, et al. Cyclic adenosine monophosphate suppresses the transcription of proinflammatory cytokines via the phosphorylated c-Fos protein. Immunity. 2009;30:372–383.
Huang B, Yang XD, Lamb A, Chen LF. Posttranslational modifications of NF-kappaB: another layer of regulation for NF-kappaB signaling pathway. Cell Signal. 2010;22:1282–1290.
Mills EL, O’Neill LA. Reprogramming mitochondrial metabolism in macrophages as an anti-inflammatory signal. Eur J Immunol. 2016;46:13–21.
Forman HJ, Torres M. Reactive oxygen species and cell signaling: respiratory burst in macrophage signaling. Am J Respir Crit Care Med. 2002;166:S4–S8.
Czapski GA, Cakala M, Kopczuk D, Strosznajder JB. Effect of poly(ADP-ribose) polymerase inhibitors on oxidative stress evoked hydroxyl radical level and macromolecules oxidation in cell free system of rat brain cortex. Neurosci Lett. 2004;356:45–48.
Pick E, Keisari Y. Superoxide anion and hydrogen peroxide production by chemically elicited peritoneal macrophages–induction by multiple nonphagocytic stimuli. Cell Immunol. 1981;59:301–318.
Aruoma OI, Halliwell B, Hoey BM, Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med. 1989;6:593–597.
Scott CL, Swisher EM, Kaufmann SH. Poly(ADP-ribose) polymerase inhibitors: recent advances and future development. J Clin Oncol. 2015;33:1397–1406.
Biro A, Vaknine H, Cohen-Armon M, et al. The effect of poly(ADP-ribose) polymerase inhibition on aminoglycoside-induced acute tubular necrosis in rats. Clin Nephrol. 2016;85:226–234.
Cover C, Fickert P, Knight TR, et al. Pathophysiological role of poly(ADP-ribose) polymerase (PARP) activation during acetaminophen-induced liver cell necrosis in mice. Toxicol Sci. 2005;84:201–208.
Lakatos P, Szabo E, Hegedus C, et al. 3-Aminobenzamide protects primary human keratinocytes from UV-induced cell death by a poly(ADP-ribosyl)ation independent mechanism. Biochim Biophys Acta. 2013;1833:743–751.
Jamil I, Symonds A, Lynch S, Alalami O, Smyth M, Martin J. Divergent effects of paracetamol on reactive oxygen intermediate and reactive nitrogen intermediate production by U937 cells. Int J Mol Med. 1999;4:309–312.
Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20:1126–1167.
Abd Elmageed ZY, Naura AS, Errami Y, Zerfaoui M. The poly(ADP-ribose) polymerases (PARPs): new roles in intracellular transport. Cell Signal. 2012;24:1–8.
Matsuzawa A, Saegusa K, Noguchi T, et al. ROS-dependent activation of the TRAF6-ASK1-p38 pathway is selectively required for TLR4-mediated innate immunity. Nat Immunol. 2005;6:587–592.
Le Page C, Sanceau J, Drapier JC, Wietzerbin J. Inhibitors of ADP-ribosylation impair inducible nitric oxide synthase gene transcription through inhibition of NF kappa B activation. Biochem Biophys Res Commun. 1998;243:451–457.
Zerfaoui M, Errami Y, Naura AS, et al. Poly(ADP-ribose) polymerase-1 is a determining factor in Crm1-mediated nuclear export and retention of p65 NF-kappa B upon TLR4 stimulation. J Immunol. 2010;185:1894–1902.
Hassa PO, Hottiger MO. The functional role of poly(ADP-ribose)polymerase 1 as novel coactivator of NF-kappaB in inflammatory disorders. Cell Mol Life Sci. 2002;59:1534–1553.
Masmoudi A, Mandel P. ADP-ribosyl transferase and NAD glycohydrolase activities in rat liver mitochondria. Biochemistry. 1987;26:1965–1969.
Ha HC, Hester LD, Snyder SH. Poly(ADP-ribose) polymerase-1 dependence of stress-induced transcription factors and associated gene expression in glia. Proc Natl Acad Sci USA. 2002;99:3270–3275.
Hassa PO, Haenni SS, Buerki C, et al. Acetylation of poly(ADP-ribose) polymerase-1 by p300/CREB-binding protein regulates coactivation of NF-kappaB-dependent transcription. J Biol Chem. 2005;280:40450–40464.
Oliver FJ, Menissier-de Murcia J, Nacci C, et al. Resistance to endotoxic shock as a consequence of defective NF-kappaB activation in poly(ADP-ribose) polymerase-1 deficient mice. EMBO J. 1999;18:4446–4454.
Wei J, Dong S, Bowser RK, et al. Regulation of the ubiquitylation and deubiquitylation of CREB-binding protein modulates histone acetylation and lung inflammation. Sci Signal. 2017;10:eaak9660.
Menon D, Coll R, O’Neill LA, Board PG. Glutathione transferase omega 1 is required for the lipopolysaccharide-stimulated induction of NADPH oxidase 1 and the production of reactive oxygen species in macrophages. Free Radic Biol Med. 2014;73:318–327.
Wang K. Molecular mechanisms of hepatic apoptosis. Cell Death Dis. 2014;5:e996.
Kanno S, Ishikawa M, Takayanagi M, Takayanagi Y, Sasaki K. Characterization of hydrogen peroxide-induced apoptosis in mouse primary cultured hepatocytes. Biol Pharm Bull. 2000;23:37–42.
Shin SM, Cho IJ, Kim SG. Resveratrol protects mitochondria against oxidative stress through AMP-activated protein kinase-mediated glycogen synthase kinase-3beta inhibition downstream of poly(ADP-ribose)polymerase-LKB1 pathway. Mol Pharmacol. 2009;76:884–895.
Jouan-Lanhouet S, Arshad MI, Piquet-Pellorce C, et al. TRAIL induces necroptosis involving RIPK1/RIPK3-dependent PARP-1 activation. Cell Death Differ. 2012;19:2003–2014.
Liu Z, Li X, Ding X, Yang Y. In silico and experimental studies of concanavalin A: insights into its antiproliferative activity and apoptotic mechanism. Appl Biochem Biotechnol. 2010;162:134–145.
Imose M, Nagaki M, Naiki T, et al. Inhibition of nuclear factor kappaB and phosphatidylinositol 3-kinase/Akt is essential for massive hepatocyte apoptosis induced by tumor necrosis factor alpha in mice. Liver Int. 2003;23:386–396.
Wang K. Molecular mechanisms of hepatic apoptosis regulated by nuclear factors. Cell Signal. 2015;27:729–738.
Kanno S, Ishikawa M, Takayanagi M, Takayanagi Y, Sasaki K. Combination acetaminophen and doxapram potentiated hepatotoxicity in mouse primary cultured hepatocytes. Methods Find Exp Clin Pharmacol. 1999;21:647–652.
Shen W, Kamendulis LM, Ray SD, Corcoran GB. Acetaminophen-induced cytotoxicity in cultured mouse hepatocytes: effects of Ca(2+)-endonuclease, DNA repair, and glutathione depletion inhibitors on DNA fragmentation and cell death. Toxicol Appl Pharmacol. 1992;112:32–40.
Acknowledgments
We are grateful to Dr. C. Daniels and M. Athamna for critical reading of the manuscript, to Dr. M. Cohen-Armon for various reagents and helpful discussions, to Dr. E. Pick and Dr. E. Bechor for the help with the superoxide assay and insightful discussions; to Dr. D. Shabat, N. Hananya and O. Green for help with fluorescence measurement; to Dr. C. Yu (Xiamen University, Xiamen, Fujian, China) for the TNFα promoter luciferase plasmid; to Dr. M. Montminy (Salk Institute, La-Jolla, CA) for the CRE-luciferase plasmid; to Dr. P. Angel (German Cancer Research Center, Heidelberg, Germany) for the AP-1-luciferase plasmid; to Dr. Ariel Munitz (TAU, Israel) for the iNOS antibody; and to Dr. Y. Ebenstein (TAU, Israel) for the EL-4 cell line.
Funding
This work was supported by the United States – Israel Binational Science Foundation [Grant 2011360 to TZ]. IF is supported by the Intramural Research Program of NIAID, NIH.
Author information
Authors and Affiliations
Contributions
JW, AB and TZ conceived and designed the study; OE, AL, HA, SK, IBN, IBD, DK, and KR performed the experiments; JW, OE, AL, HA, SK, DK, OB, KR, IF, RW, AB, and TZ analyzed the data; JW, OE, AB, and TZ wrote the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
All animal experiments were performed in accordance with the guidelines of the Care and Use of Laboratory Animals and have been approved by the research ethics committee at Wolfson medical center.
Rights and permissions
About this article
Cite this article
Wardi, J., Ernst, O., Lilja, A. et al. 3-Aminobenzamide Prevents Concanavalin A-Induced Acute Hepatitis by an Anti-inflammatory and Anti-oxidative Mechanism. Dig Dis Sci 63, 3382–3397 (2018). https://doi.org/10.1007/s10620-018-5267-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-018-5267-1