Abstract
Background and Aims
Mucosal healing (MH) with thiopurines has been poorly investigated in ulcerative colitis (UC). We aimed to assess MH rate in UC patients treated with thiopurine monotherapy.
Patients and Methods
We retrospectively collected all UC patients treated with thiopurines more than 6 months who have undergone colonoscopy at baseline and after at least 6 months of treatment. Patients were recruited from January 2005 to May 2015 through a personal database and/or standardized hospital inpatient diagnostic dataset. Patients were excluded in case of any use of other immunomodulator or biological agent. MH was defined as a Mayo endoscopic subscore ≤1 and UCEIS ≤ 2. Histological healing (HH) was defined by the absence of epithelial polynuclear infiltrate, cryptic abscesses, or ulcerations.
Results
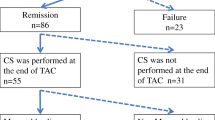
Eighty patients (31 women, median age 43 [IQR 32–58]) were included. Median disease duration was 10.5 [6–16] years. At baseline, median full Mayo score, endoscopic subscore, and UCEIS were 8 [6.8–10], 3 [2–3], and 5 [3–6], respectively. MH was first assessed after a mean follow-up of 38 ± 31 months. Median full Mayo score, endoscopic subscore, and UCEIS decreased to 3.5 [1–6], 2 [0–2.2], and 2 [0–4], respectively. MH was achieved in 43.7%, HH in 38%. In multivariate analysis, predictors of MH were thiopurine exposure duration ≥2 years [odds ratio (OR) 2.9, CI 95% (1.1–7.6), p = 0.03] and a prior acute severe colitis [OR 5.9, CI 95% (1.1–32), p = 0.04]. Factors associated with MH during treatment were partial Mayo score ≤2 (NPV = 100%), BMI ≥ 25 kg/m2 (NPV = 75%), and MCV ≥ 95 fL (NPV = 73%).
Conclusions
In UC, thiopurine monotherapy is associated with MH in 43.7% and HH in 38%.

Similar content being viewed by others
Abbreviations
- UC:
-
Ulcerative colitis
- MH:
-
Mucosal healing
- HH:
-
Histological healing
- SD:
-
Standard deviation
- PPV:
-
Positive predictive value
- NPV:
-
Negative predictive value
- MCV:
-
Mean corpuscular volume
- BMI:
-
Body mass index
- IQR:
-
Interquartile range
References
Klotz C, Barret M, Dhooge M, et al. Management of diagnosis and treatment in ulcerative colitis. Presse Méd. 2015;44:144–149.
Dignass A, Lindsay JO, Sturm A, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis. 2012;6:991–1030.
López-Palacios N, Mendoza JL, Taxonera C, et al. Mucosal healing for predicting clinical outcome in patients with ulcerative colitis using thiopurines in monotherapy. Eur J Intern Med. 2011;22:621–625.
Colombel JF, Rutgeerts P, Reinisch W, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011;141:1194–1201.
Laharie D, Filippi J, Roblin X, et al. Impact of mucosal healing on long-term outcomes in ulcerative colitis treated with infliximab: a multicenter experience. Aliment Pharmacol Ther. 2013;37:998–1004.
Gupta RB, Harpaz N, Itzkowitz S, et al. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology. 2007;133:1099–1105.
Rutter M, Saunders B, Wilkinson K, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451–459.
Peyrin-Biroulet L, Bressenot A, Kampman W. Histologic remission: the ultimate therapeutic goal in ulcerative colitis? Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2014;12:929–934.
Kruis W, Kiudelis G, Rácz I, et al. Once daily versus three times daily mesalazine granules in active ulcerative colitis: a double-blind, double-dummy, randomised, non-inferiority trial. Gut. 2009;58:233–240.
Bokemeyer B, Hommes D, Gill I, et al. Mesalazine in left-sided ulcerative colitis: efficacy analyses from the PODIUM trial on maintenance of remission and mucosal healing. J Crohns Colitis. 2012;6:476–482.
Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476.
Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142:257–265.
Ardizzone S, Maconi G, Russo A, et al. Randomised controlled trial of azathioprine and 5-aminosalicylic acid for treatment of steroid dependent ulcerative colitis. Gut. 2006;55:47–53.
Timmer A, McDonald JWD, Tsoulis DJ, et al. Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2012;9:CD000478.
Yamada S, Yoshino T, Matsuura M, et al. Efficacy and safety of long-term thiopurine maintenance treatment in japanese patients with ulcerative colitis. Intest Res. 2015;13:250–258.
Panaccione R, Ghosh S, Middleton S, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology. 2014;146:392–400.
Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19:5A–36A.
Lewis JD, Chuai S, Nessel L, et al. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. 2008;14:1660–1666.
Travis SPL, Schnell D, Krzeski P, et al. Developing an instrument to assess the endoscopic severity of ulcerative colitis: the Ulcerative Colitis Endoscopic Index of Severity (UCEIS). Gut. 2012;61:535–542.
Bressenot A, Salleron J, Bastien C, et al. Comparing histological activity indexes in UC. Gut. 2015;64:1412–1418.
D’Haens G, Sandborn WJ, Feagan BG, et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. 2007;132:763–786.
Sutherland L, Roth D, Beck P, et al. Oral 5-aminosalicylic acid for maintaining remission in ulcerative colitis. Cochrane Database Syst Rev. 2000;CD000544. doi:10.1002/14651858.CD000544.
Sandborn WJ, Feagan BG, PURSUIT-Maintenance Study Group, et al. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146:96–109.
Pineton de Chambrun G, Peyrin-Biroulet L, Lémann M, et al. Clinical implications of mucosal healing for the management of IBD. Nat Rev Gastroenterol Hepatol. 2010;7:15–29.
Travis SPL, Schnell D, Krzeski P, et al. Reliability and initial validation of the ulcerative colitis endoscopic index of severity. Gastroenterology. 2013;145:987–995.
Travis SPL, Schnell D, Feagan BG, et al. The impact of clinical information on the assessment of endoscopic activity: characteristics of the Ulcerative Colitis Endoscopic Index Of Severity [UCEIS]. J Crohns Colitis. 2015;9:607–616.
Feagan BG, Rutgeerts P, GEMINI 1 Study Group, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699–710.
Reinisch W, Sandborn WJ, Hommes DW, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut. 2011;60:780–787.
Manginot C, Baumann C, Peyrin-Biroulet L. An endoscopic Mayo score of 0 is associated with a lower risk of colectomy than a score of 1 in ulcerative colitis. Gut. 2014;64:1181–1182.
Yokoyama K, Kobayashi K, Mukae M, et al. Clinical study of the relation between mucosal healing and long-term outcomes in ulcerative colitis. Gastroenterol Res Pract. 2013;2013:192794. doi:10.1155/2013/192794.
Bryant RV, Burger DC, Delo J, et al. Beyond endoscopic mucosal healing in UC: histological remission better predicts corticosteroid use and hospitalisation over 6 years of follow-up. Gut. 2015;65:408–414.
Chhaya V, Saxena S, Cecil E, et al. The impact of timing and duration of thiopurine treatment on colectomy in ulcerative colitis: a national population-based study of incident cases between 1989–2009. Aliment Pharmacol Ther. 2015;41:87–98.
Sood R, Ansari S, Clark T, et al. Long-term efficacy and safety of azathioprine in ulcerative colitis. J Crohns Colitis. 2015;9:191–197.
Solberg IC, Lygren I, Jahnsen J, et al. Clinical course during the first 10 years of ulcerative colitis: results from a population-based inception cohort (IBSEN Study). Scand J Gastroenterol. 2009;44:431–440.
Lee H-S, Yang S-K, Soh JS, et al. Short- and long-term outcomes of acute severe ulcerative colitis in Korea: the 1999–2005 Cohort. Inflamm Bowel Dis. 2015;21:1825–1831.
O’Byrne S, Keir ME, Cabanski CR et al. Rectal bleeding accurately reflects level of mucosal inflammation in patients with ulcerative colitis. Gastroenterology. 2015; 4:Su 1220.
Bouguen G, Sninsky C, Tang KL, et al. Change in erythrocyte mean corpuscular volume during combination therapy with azathioprine and infliximab is associated with mucosal healing: a post hoc analysis from SONIC. Inflamm Bowel Dis. 2015;21:606–614.
Thapa SD, Hadid H, Usman M, et al. Predictors of thiopurine treatment failure in biologic-naïve ulcerative colitis patients. Dig Dis Sci. 2016;61:230–237.
Sahasranaman S, Howard D, Roy S. Clinical pharmacology and pharmacogenetics of thiopurines. Eur J Clin Pharmacol. 2008;64:753–767.
Author contributions
Caroline Prieux-Klotz contributed to study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis, and technical or material support. Stephane Nahon participated in study concept and design, acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, and technical or material support. Aurélien Amiot involved in acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, statistical analysis, and technical or material support. Leila Sinayoko and Carole Galéano-Cassaz analyzed and interpreted the data. Stanislas Chaussade and Romain Coriat participated in critical revision of the manuscript for important intellectual content and technical or material support. Pierre Lahmek involved in statistical analysis. Vered Abitbol contributed to study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis, technical or material support, and study supervision.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare in connection with this paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Prieux-Klotz, C., Nahon, S., Amiot, A. et al. Rate and Predictors of Mucosal Healing in Ulcerative Colitis Treated with Thiopurines: Results of a Multicentric Cohort Study. Dig Dis Sci 62, 473–480 (2017). https://doi.org/10.1007/s10620-016-4374-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-016-4374-0