Abstract
Objective
Few studies have assessed the loss of efficacy or patient and caregiver satisfaction with rapid infliximab infusions. The aim of this study is to assess the tolerability, loss of efficacy and to describe the impact on resource utilization and patient satisfaction in rapid infliximab infusions.
Methods
Subjects with inflammatory bowel disease receiving rapid infliximab infusions were included in the study. Subjects received maintenance infusions from June 2011 to June 2013. Incidence of adverse reactions and the total number of rapid infliximab infusions were recorded. Efficacy was compared to published studies evaluating the long-term efficacy of infliximab infusions. Patient satisfaction was addressed through a survey following the implementation of the rapid infusion protocol.
Results
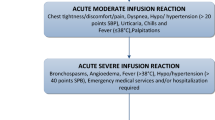
Seventy-five subjects with IBD were included in the study. Five hundred and twenty-two rapid infliximab infusions were provided to patients. There were no acute or delayed infusion reactions. Ten subjects (13 %) required either a dose escalation or interval adjustment between infliximab infusions. A majority of patients reported increased satisfaction with 1-h infliximab infusions, and 97 % of surveyed patients opted to continue rapid infusions. The rapid infliximab infusion protocol increased infusion unit efficiency by increasing capacity by 15 %. Cost savings in the elimination of nursing time translated to approximately $108,150 savings at our institution.
Conclusions
Rapid infliximab infusions do not appear to increase the risk of loss of response compared to historical studies of long-term infliximab efficiency. A rapid infliximab infusion protocol improved efficiency in our infusion unit and increased patient and nursing satisfaction.


Similar content being viewed by others

References
Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476.
Targan SR, Hanauer SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med. 1997;337:1029–1035.
Hanauer SB, Feagan BG, Lichtenstein GR, et al. ACCENT I Study Group. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549.
Sands BE, Blank MA, Patel K, van Deventer SJ. Long-term treatment of rectovaginal fistulas in Crohn’s disease: response to infliximab in the ACCENT II Study. Clin Gastroenterol Hepatol. 2004;2:912–920.
Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350:876–885.
Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med. 1999;340:1398–1405.
Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105:501–523.
Lichtenstein GR, Hanauer SB, Sandborn WJ. Management of Crohn’s disease in adults. Am J Gastroenterol. 2009;104:465–483.
Gramlick A, Fossati G, Nesbitt AM. Neutralization of soluble and membrane tumor necrosis factor-alpha (TNF- alpha) by infliximab, adalimumab, or certolizumab pegol using P55 or P75 TNF-alpha receptor specific bioassays. Gastroenterology. 2006;130:A697.
Mitoma H, Horiuchi T, Tsukamoto H. Binding activities of infliximab and etanercept to transmembrane tumor necrosis factor-alpha. Gastroenterology. 2004;26:934–935.
Lugering A, Schmidt M, Lugering N, Pauels HG, Domschke W, Kucharzik T. Infliximab induces apoptosis in monocytes from patients with chronic active Crohn’s disease by using a caspase-dependent pathway. Gastroenterology. 2001;121:1145–1457.
Cheifetz A, Smedley M, Martin S, et al. The incidence and management of infusion reactions to infliximab: a large center experience. Am J Gastroenterol. 2003;98:1315–1324.
Schaible TF. Long term safety of infliximab. Can J Gastroenterol. 2000;14:29C–32C.
van Vollenhoven RF, Gullstrom E, Klareskog L. Feasibility of 1 h infliximab infusions. Ann Rheum Dis. 2005;64:654.
Buch MH, Bryer D, Lindsay S, et al. Shortening infusion times for infliximab administration. Rheumatology. 2006;45:485–486.
Salzberg B, Kendall K. Successful 1 h infusions of infliximab for Crohn’s disease patients in an outpatient office setting. Gastroenterology. 2004;126:A629.
Befrits R, Malmström L, Forsell A, et al. One hour infliximab infusions can replace 2 h infusion in maintenance treatment of Crohn’s disease without shortcomings, in patients treated for up to 3 years. Gastroenterology. 2008;134:A402.
Bhat S, Sharma D, et al. Are accelerated infliximab infusions safe in patients with inflammatory bowel disease? Inflamm Bowel Dis. 2010;16:1922–1925.
Clare DF, Alexander FC, Mike S, et al. Accelerated infliximab infusions are safe and well tolerated in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2009;21:71–75.
Van Assche G, Vermeire S, Noman M, et al. Infliximab administered with shortened infusion times in a specialized IBD infusion unit: a prospective cohort study. J Crohns Colitis. 2010;4:329–333.
Barbouri A, Buisson A, Bigard MA, et al. Tolerability of 1 h 10 mg/kg infliximab infusions in patients with inflammatory bowel disease. J Crohns Colitis. 2013;7:129–133.
Breynaert C, Ferrante M, Fidder H, et al. Tolerability of shortened infliximab infusion times in patients with inflammatory bowel diseases: a single-center cohort study. Am J Gastroenterology. 2011;106:778–785.
Neef HC, Riebschleger MP, Adler J. Meta-analysis: rapid infliximab infusions are safe. Aliment Pharmacol Ther. 2013;38:365–376.
Reinisch W, Sandborn W, Rutgeerts P, et al. Long-term infliximab maintenance therapy for ulcerative colitis: the ACT-1 and -2 extension studies. Inflamm Bowel Dis. 2012;18:201–211.
Rostholder E, Ahmed A, Cheifetz S, et al. Outcomes after escalation of infliximab therapy in ambulatory patients with moderately active ulcerative colitis. Aliment Pharmacol Ther. 2012;35:562–567.
Taxonera C, Barreiro-de Acosta M, Calvo M, et al. Short- and long-term outcomes of infliximab dose intensification in patients with ulcerative colitis. Gastroenterology. 2012;142:S355.
Lazarev M, Ullman T, Mayer L, et al. The need for infliximab dose escalation in ulcerative colitis [abstract]. Am J Gastroenterol. 2009;104:S440.
Regueiro M, Siemanowski B, Kip K, et al. Infliximab dose intensification in Crohn’s disease. Inflamm Bowel Dis. 2007;13:1093–1099.
Shih CE, Bayless TM, Harris ML. Maintenance of long term response to infliximab over 1–5 years in Crohn’s Disease including shortening dosing intervals or increasing dosage. Gastroenterology. 2004;126:A631.
Thameem D, Kugathasan S, Hatoum OA, et al. Acceleration of infliximab maintenance therapy in Crohn’s Disease. Gastroenterology. 2005;128:A587.
Corman S, Issa M, Beaulieu DB, et al. Escalation of infliximab maintenance therapy in Crohn’s Disease. Am J Gastroenterol. 2006;101:S470.
Ungar B, Chowers Y, Yavzori M, et al. The temporal evolution of antidrug antibodies in patients with inflammatory bowel disease treated with infliximab. Gut. 2014;63:1258–1264.
Gunnarsson C, Chen J, Rizzo JA, et al. Direct health care insurer and out-of-pocket expenditures of inflammatory bowel disease: evidence from a national survey. Dig Dis Sci. 2012;57:3080–3091.
Park KT, Bass D. Inflammatory bowel disease-attributable costs and cost-effective strategies in the United States: a review. Inflamm Bowel Dis. 2011;17:1603–1609.
Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long -term efficacy of infliximab in Crohn’s disease. N Engl J Med. 2003;348:601–608.
Nanda KS, Cheifetz AS, Moss AC. Impact of antibodies to infliximab on clinical outcomes and serum infliximab levels in patients with inflammatory bowel disease (IBD): a meta-analysis. Am J Gastroenterol. 2013;108:40–47.
CMS-1601-FC Medicare and Medicaid Programs: Hospital Outpatient Prospective Payment and Ambulatory Surgical Center Payment Systems and Quality Reporting Programs; Final Rule with Comment Period, Addendum B; displayed November 27, 2013, at: http://www.ofr.gov/OFRUpload/OFRData/2013-28737_PI.pdf
Terdiman JP, Gruss CB, Heidelbaugh JJ, et al. American gastroenterological association institute guideline on the use of thiopurines, methotrexate, and anti-TNF-α biologic drugs for the induction and maintenance of remission in inflammatory Crohn’s disease. Gastroenterology. 2013;145:1459–1463.
Dignass A, Linday JO, Sturm A, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis Part 2: current management. J Crohn’s Colitis. 2012;6:991–1030.
Dignass A, Van Assche G, Lindsay J, et al. The second European evidence-based consensus on the diagnosis and management of Crohn’s disease: current Management. J Crohn’s Colitis. 2010;4:28–62.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Qazi, T., Shah, B., El-Dib, M. et al. The Tolerability and Efficacy of Rapid Infliximab Infusions in Patients with Inflammatory Bowel Disease. Dig Dis Sci 61, 589–596 (2016). https://doi.org/10.1007/s10620-015-3893-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-015-3893-4