Abstract
Background and aims
Neurensin-2 (NRSN2) is a neuronal membrane protein; previous reports indicated that it might function as a tumor suppressor in hepatocellular carcinoma (HCC). However, its biological functions and associated mechanisms remain unknown. In the current study, we aimed to investigate the biological functions and possible mechanisms of neurensin-2.
Methods
The mRNA and protein level of NRSN2 in HCC has tissues and cell lines were detected by quantitative real-time PCR, immunohistochemistry staning and western blot. Overexpressing and silencing the level of NRSN2 in HCC cell lines were used to investigate the role of NRSN2 in HCC. CCK-8 assays, SA-β gel staining, Annexin V/PI staining, quantitative real-time PCR and western blot were employed to explore the role and mechanisms of HCC.
Results
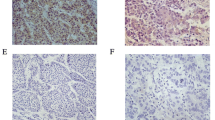
NRSN2 was more commonly down-regulated HCC tissues compared with adjacent tissues, and the expression pattern of NRSN2 was not only closely correlated with tumor size and TNM stage but also negatively correlated with patient prognosis. Both loss and gain function assays revealed that NRSN2 inhibits cancer cell proliferation and promotes cancer cell senescence and apoptosis. We further found that NRSN2 might regulate PI3K/AKT signaling and p53/p21 pathway to exert its role in HCC cell proliferation, senescence and apoptosis.
Conclusion
Our study validates the suppressive role of NRSN2 in both clinicopathologic and biological aspects in HCC tumorigenesis.





Similar content being viewed by others
References
Parkin DM. Global cancer statistics in the year 2000.
Poon RT-P, Fan S-T, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232:10–24.
Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment nature reviews. Cancer. 2006;6:674–687.
Yang JD, Nakamura I, Roberts LR. The tumor microenvironment in hepatocellular carcinoma: current status and therapeutic targets. Semin Cancer Biol. 2011;21:35–43.
Zender L, Xue W, Zuber J, et al. An oncogenomics-based in vivo RNAi screen identifies tumor suppressors in liver cancer. Cell. 2008;135:852–864.
Nakanishi K, Ida M, Suzuki H, et al. Molecular characterization of a transport vesicle protein Neurensin-2, a homologue of Neurensin-1, expressed in neural cells. Brain Res. 2006;1081:1–8.
An Y, Amr SS, Torres A, et al. SOX12 and NRSN2 are candidate genes for 20p13 subtelomeric deletions associated with developmental delay. Am J Med Genet Part B Neuropsychiatr Genet. 2013;162b:832–840.
Ma HQ, Liang XT, Zhao JJ, et al. Decreased expression of Neurensin-2 correlates with poor prognosis in hepatocellular carcinoma. World J Gastroenterol. 2009;15:4844–4848.
Feng MX, Ma MZ, Fu Y, et al. Elevated autocrine EDIL3 protects hepatocellular carcinoma from anoikis through RGD-mediated integrin activation. Mol Cancer. 2014;13:226.
Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326.
Dent P. Crosstalk between ERK, AKT, and cell survival. Cancer Biol Ther. 2014;15:245–246.
Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522.
Pelicci PG. Do tumor-suppressive mechanisms contribute to organism aging by inducing stem cell senescence? J Clin Investig. 2004;113:4–7.
Jackson JG, Pereira-Smith OM. p53 is preferentially recruited to the promoters of growth arrest genes p21 and GADD45 during replicative senescence of normal human fibroblasts. Cancer Res. 2006;66:8356–8360.
Morgensztern D, McLeod HL. PI3K/Akt/mTOR pathway as a target for cancer therapy. Anticancer Drugs. 2005;16:797–803.
Zhou Q, Lui VW, Yeo W. Targeting the PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Future Oncol. 2011;7:1149–1167.
Zhou L, Huang Y, Li J, Wang Z. The mTOR pathway is associated with the poor prognosis of human hepatocellular carcinoma. Med Oncol. 2010;27:255–261.
Reed JC. Bcl-2 family proteins. Oncogene. 1998;17:3225–3236.
Coultas L, Strasser A. The role of the Bcl-2 protein family in cancer. Semin Cancer Biol. 2003;13:115–123.
Wang J-M, Chao J-R, Chen W, Kuo M-L, Yen JJ-Y, Yang-Yen H-F. The antiapoptotic gene mcl-1 is up-regulated by the phosphatidylinositol 3-kinase/Akt signaling pathway through a transcription factor complex containing CREB. Mol Cell Biol. 1999;19:6195–6206.
Mooi WJ, Peeper DS. Oncogene-induced cell senescence—halting on the road to cancer. N Engl J Med. 2006;355:1037–1046.
Roninson IB. Tumor cell senescence in cancer treatment. Cancer Res. 2003;63:2705–2715.
Gottlieb TM, Leal JF, Seger R, Taya Y, Oren M. Cross-talk between Akt, p53 and Mdm2: possible implications for the regulation of apoptosis. Oncogene. 2002;21:1299–1303.
Acknowledgments
This work was supported by grants from the Training Program for Superb Academic Leaders in Shanghai Health System (No. XBR2011029). We thank the Shanghai Special Fund for Building Leading Talent Teams.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Xin Wang and Longzhi Han contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, X., Han, L., Zhang, J. et al. Down-Regulated NRSN2 Promotes Cell Proliferation and Survival Through PI3K/Akt/mTOR Pathway in Hepatocellular Carcinoma. Dig Dis Sci 60, 3011–3018 (2015). https://doi.org/10.1007/s10620-015-3736-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-015-3736-3