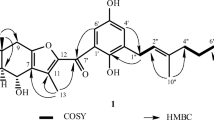
A phytochemical investigation of the ethyl acetate fraction of root bark extract of Synadenium glaucescens Pax (Euphorbiaceae) led to the isolation of two derivatives of ellagic acid. These included one new 3′,4′ -di-O-methylellagic acid-4α, L-rhamnopyranoside (1) and a known 3,4,3′-tri-O-methylellagic acid (2). Their structures were elucidated by 1D and 2D NMR, while a High res. MS (FTMS-ESI–) was used for accurate mass measurement, which was compared with SciFinder data as well as with available literature. The BSLT results indicated that both 1 (LC50 = 2736.03 μg/mL) and 2 (LC50 = 531.19 μg/mL) were nontoxic at a maximum test concentration of 2400 ppm.

Similar content being viewed by others
References
P. M. Cheuka, G. Mayoka, P. Mutai, and K. Chibale, Molecules, 22, 1 (2017).
D. B. Shelar and P. J. Shirote, Biomed. Pharmacol. J., 4, 141 (2011).
R. Emily, Medicinal Plants at Risk,Vol. 1, Centre for Biological Diversity, Tucson, 2008, 19 pp.
L. K. M. Merlin, K. Gustav, A. D. Forkuo, C. Firempong, A. K. Anning, and D. A. Rita, IntechOpen, 64 (2019).
S. M. K. Rates, Toxicon, 39, 603 (2001).
N. Abubakar, K. Shehu, M. M. Yahaya, I. Y. Tafinta, and M. A. Imonikhe, Ann. Biol. Sci., 4, 26 (2016).
T. J. Mwine and P. van Damme, J. Med. Plants Res., 5, 652 (2011).
F. P. Mabiki, R. H. Mdegela, R. D. Mosha, and J. J. Magadula, J. Med. Plants Res., 7, 871 (2013).
V. A. Nyigo, H. M. Malebo, F. Mabiki, and R. Mdegela, JPHYTO, 11, 151 (2022).
V. A. Nyigo, X. Peter, F. Mabiki, H. M. Malebo, R. H. Mdegela, and G. Fouche, J. Phytopharm. JPHYTO, 5, 100 (2016).
D. Credo, F. P. Mabiki, F. Machumi, M. Chacha, C. Cornett, and B. Styrishave, Trop. Biomed., 39, 1 (2022).
Atta-Ur-Rahman, F. N. Ngounou, C. M. Iqbal, M. Shahid, M. Talat, M. Nur-E-Alam, Z. Seema, D. Lontsi, J. F. Ayafor, and B. L. Songendam, Planta Med., 67, 335 (2001).
J. P. Kim, I. K. Lee, B. S. Yun, S. H. Chung, G. S. Shim, H. Koshino, and Y. Ick-Dong, Phytochemistry, 57, 587 (2001).
J. L. Ríos, R. M. Giner, M. Marin, and M. C. Recio, Planta Med., 84, 1068 (2018).
J. L. Maas, G. J. Galletta, and G. D. Stoner, HortScience, 26, 10 (2019).
H. A. Bedel, C. K. Manas, G. Ozbey, and C. Usta, Nat. Prod. Res., 32, 2932 (2018).
M. M. Kannan and S. D Quine, Metabolism, 62, 52 (2013).
J. B. R. Jordao, H. K. P. Porto, F. M. Lopes, A. C. Batista, and M. L. Rocha, Planta Med., 83, 830 (2017).
A. J. Amor, C. Gomez-Guerrero, E. Ortega, A. Sala-Vila, and I. Lazaro, Antioxidants, 9, 1 (2020).
B. Prabha, S. Sini, T. S. Priyadarshini, P. Sasikumar, G. Gopalan, J. P. Joseph, M. M. Jithin, V. Sivan, P. Jayamurthy, and K. V. Radhakrishnan, Nat. Prod. Res., 35, 3151 (2019).
P. F Uzor and P. O. Osadebe, EXCLI J., 15, 290 (2016).
M. Saadullah, M. Asif, A. Sattar, K. Rehman, S. Shah, M. Saleem, A. Shah, M. Wajid, A. Rasool, M. Uzair, and K. Afzal, Trop. J. Pharm. Res., 19, 1073 (2020).
H. Zhang, Z. J. Guo, W. M. Xu, X. J. You, L. Han, Y. X. Han, and L. J. Dai, Oncol. Lett., 7, 525 (2014).
J. D. Djoukeng, E. Abou-Mansour, L. A. Tapondjou, D. Lontsi, and R. Tabacchi, Nat. Prod. Commun., 2, 261 (2007).
S. A. A. El-Toumy and H. W. Rauwald, Planta Med., 69, 682 (2003).
Q. Guo and X. Yang, Pharmazie, 60, 60 (2005).
E. Dagne, M. Alemu, and O. Sterner, Bull. Chem. Soc. Ethiop., 7, 87 (1993).
G. Ye, H. Peng, M. Fan, and C. G. Huang, Chem. Nat. Compd., 43, 125 (2007).
Z. Z. Ibraheim, A. S. Ahmed, and W. M. Abdel-Mageed, J. Nat. Remedies, 13, 35 (2013).
S. Begum, Sara, S. Tauseef, B. Sh. Siddiqui, Sh. S. Nizami, H. Ghulam, and A. Ahmad, J. Chem. Soc. Pakistan, 36, 723 (2014).
T. Tukiran, A. P. Wardana, N. Hidayati, and K. Shimizu, Indones. J. Chem., 18, 26 (2018).
A. Hiranrat, Doctoral Thesis in Organic Chemistry, Prince of Songkla Uni. Hat Yai, 2010, 257 pp.
B. N. Meyer, N. R. Ferrigni, J. E. Putnam, L. B. Jacobsen, D. E. Nichols, and J. L. McLaughlin, Planta Med., 45, 31 (1982).
M. J. Moshi, E. Innocent, J. J. Magadula, D. F. Otieno, A. Weisheit, P. K. Mbabazi, and R. S. O. Noando, Tanzan J. Health Res., 12, 7 (2010).
Acknowledgment
The authors acknowledge the financial support from Green Resources Innovations for Livelihood Improvement (GRILI-DANIDA) project (Grant No. 18-3-TAN). Also Mr. Christopher Johnson Mwankuna from the Department of Chemistry and Physics, Sokoine University of Agriculture, Morogoro, Tanzania and Mr. Christian Janflet from the Department of Pharmacy, University of Copenhagen, Denmark are acknowledged for their technical support. The NMR equipment used in this work was purchased via Grant No. 10-085264 from The Danish Research Council for Independent Research, Nature and Universe.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 4, July–August, 2023, pp. 564–567
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rwegoshora, F., Mabiki, F., Machumi, F. et al. A New Ellagic Acid Rhamnoside from Synadenium glaucescens and Its Cytotoxicity Evaluation. Chem Nat Compd 59, 670–674 (2023). https://doi.org/10.1007/s10600-023-04083-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-023-04083-8