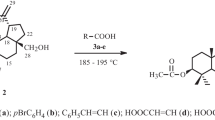
Primary and secondary triterpene alcohols were acetylated for the first time using tetraacetylglycoluril (TAGU). Cholesterol, allobetulin, and betulin acetates were obtained in high yields. The acetylation used p-TsOH or TFA in refluxing CHCl3. TFA was found to be an effective acetylation catalyst.
Similar content being viewed by others
References
F. E. Koehn and G. T. Carter, Nat. Rev. Drug Discovery, 4 (3), 206 (2005).
J. W. H. Li and J. C. Vederas, Science, 325, 161 (2009).
H. Hagiwara, K. Morohashi, H. Sakai, T. Suzuki, and M. Ando, Tetrahedron, 54, 5845 (1998).
F. Shirini, M. A. Zolfigol, and S. Mallakpour, Russ. J. Org. Chem., 41, 625 (2005).
F. Shirini, M. A. Zolfigol, M. Abedini, and P. Salehi, Bull. Korean Chem. Soc., 24, 1683 (2003).
C. W. Lee, H. Y. Hwang, H. M. Jeong, U. C. Yoon, and K. W. Chi, Synth. Met., 159, 1820 (2009).
K. Niknam and D. Saberi, Tetrahedron Lett., 50, 5210 (2009).
D. Kuhling, Ann. Chem., 263 (1973).
C. Hase and D. Kuhling, Ann. Chem., 95 (1975).
a) C. M. Tice and B. Ganem, J. Org. Chem., 48, 2106 (1983); b) J. Kalisiak, S. A. Trauger, E. Kalisiak, H. Morita, V. V. Fokin, M. W. W Adams, K. B. Sharpless, and G. Siuzdak, J. Am. Chem. Soc., 131, 378 (2009).
B. Danieli, M. Luisetti, S. Riva, A. Bertinotti, E. Ragg, and L. Scaglioni, J. Org. Chem., 60, 3637 (1995).
S. Gebhardt, S. Bihler, M. Schubert-Zsilavecz, S. Riva, D. Monti, F. Laura, and B. Danieli, Helv. Chim. Acta, 85, 1943 (2002).
B. Yu, G. Xing, Y. Hui, and X. Han, Tetrahedron Lett., 42, 5513 (2001).
B. Danieli, G. Lesma, M. Luisetti, and S. Riva, Tetrahedron, 53, 5855 (1997).
S. Arrous, A. Bakibaev, P. Hoang, I. Boudebouz, and V. Malkov, Int. J. ChemTech. Res., 11, 285 (2018).
W. F. Bruce and J. O. Ralls, Organic Synthesis, Vol. 2, John Wiley & Sons, New York, 1950, 193 pp.
J. Klinot and A. Vystrcil, Collect. Czech. Chem. Commun., 29, 516 (1964).
Acknowledgment
The work was sponsored by the Ministry of Education and Science of the Russian Federation (Grant No. 05-13108).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 3, May–June, 2019, pp. 412–414.
Rights and permissions
About this article
Cite this article
Arrous, S., Boudebouz, I., Bakibaev, A. et al. Novel Method for O-Acetylation of Cholesterol, Allobetulin, and Betulin. Chem Nat Compd 55, 482–484 (2019). https://doi.org/10.1007/s10600-019-02720-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-019-02720-9