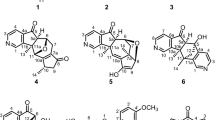
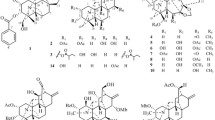
New derivatives of the diterpene alkaloid lappaconitine containing a 1H-substituted 1,2,3-triazol-4-yl substituent in the anthranilate C-5′-position were synthesized via the reaction of 5′-ethynyllappaconitine with aromatic or aliphatic azides in the presence of aqueous CuSO4 and sodium ascorbate in DMF. The yields of the 1,4-disubstituted 1,2,3-triazoles were 40–73% and depended on the nature of the azide.
Similar content being viewed by others
References
V. E. Romanov, G. R. Sabankulova, M. M. Shakirov, and E. E. Shul′ts, Zh. Org. Khim., 50, 979 (2014).
F. N. Dzhakhangirov, M. N. Sultankhodzhaev, B. Tashkhodzhaev, and B. T. Salimov, Chem. Nat. Compd., 33, 190 (1997); M. S. Yunusov, Izv. Ross. Akad. Nauk, Ser. Khim., 620 (2011).
T. G. Tolstikova, E. E. Shul′ts, A. O. Bryzgalov, M. V. Khvostov, V. E. Romanov, S. A. Osadchii, and G. A. Tolstikov, Khim. Interesakh Ustoich. Razvit., 15, 599 (2007).
A. O. Bryzgalov, V. E. Romanov, T. G. Tolstikova, and E. E. Shults, Cardiovasc. Hematol. Agents Med. Chem., 11, 211 (2013).
J. Zhang, H. Zhang, W.-X. Cai, L.-P. Yu, X.-C. Zhen, and A. Zhang, Bioorg. Med. Chem., 17, 4873 (2009).
A. Lauria, R. Delishi, F. Mingoia, A. Terenzi, A. Martorana, G. Barone, and A. M. Almerico, Eur. J. Org. Chem., 3289 (2014).
V. V. Rostovtsev, L. G. Green, V. V. Fokin, and K. B. Sharpless, Angew. Chem., Int. Ed., 41, 2596 (2002).
A. Tahghighi, S. Razmi, M. Mahdavi, P. Foroumadi, S. K. Ardestani, S. Emami, F. Kobarfard, S. Dastmalchi, A. Shafiee, and A. Foroumadi, Eur. J. Med. Chem., 50, 124 (2012).
A. Krasinski, Z. Radic, R. Manetsch, J. Raushel, P. Taylor, K. B. Sharpless, and H. C. Kolb, J. Am. Chem. Soc., 127, 6686 (2005).
A. Shi, L. Huang, C. Lu, F. He, and X. Li, Bioorg. Med. Chem., 19, 2298 (2011).
J. Mareddy, S. B. Nallapati, J. Anireddy, Y. P. Devi, L. N. Mangamoori, R. Kapavarapu, and S. Pal, Bioorg. Med. Chem. Lett., 23, 6721 (2013).
S. A. Osadchii, E. E. Shul′ts, E. V. Polukhina, V. G. Vasil′ev, and G. A. Tolstikov, Dokl. Akad. Nauk, 416, 251 (2007).
O. Berger, A. Kaniti, C. Tran van Ba, et al., ChemMedChem, 6, 2094 (2011).
C. Courme, S. Gillon, N. Gresh, and M. Vidal, Tetrahedron Lett., 49, 4542 (2008).
C. Rossy, J. Majimel, M. Delapierre, and E. Fouquet, J. Organomet. Chem., 755, 78 (2014).
F. Himo, T. Lovell, R. Hilgraf, V. V. Rostovtsev, L. Noodleman, K. B. Sharpless, and V. V. Fokin, J. Am. Chem. Soc., 127, 210 (2005).
P. Froeyen, Phosphorus Sulfur Silicon Relat. Elem., 102, 253 (1995).
A. E. Prosenko, A. A. Skorobogatov, O. I. Dyubchenko, P. I. Pinko, N. V. Kandalintseva, M. M. Shakirov, and L. M. Pokrovsky, Russ. Chem. Bull., 56, 1119 (2007).
M. Ciufolini, S. Canesi, and M. Ousmer, Tetrahedron, 62, 5318 (2006).
T. L. Amyes and W. P. Jencks, J. Am. Chem. Soc., 111, 7900 (1989).
S. Liu, B. Zhou, and H. Yang, J. Am. Chem. Soc., 130, 8251 (2008).
N. Rosario-Amorin, T. Gaboyard, G. Clerac, and M. Vellutini, Chem. Eur. J., 18, 3305 (2012).
H. Liang and M. Ciufolini, Chem. Eur. J., 16, 13262 (2010).
Acknowledgment
The work was sponsored by Russian Scientific Foundation Grant No. 14-13-00822. We thank A. P. Krysin for supplying samples of the starting phenolic compounds.
Author information
Authors and Affiliations
Corresponding author
Additional information
*For No. XX, see [1].
Translated from Khimiya Prirodnykh Soedinenii, No. 6, November–December, 2015, pp. 982–985.
Rights and permissions
About this article
Cite this article
Romanov, V.E., Shul′ts, E.E. Alkaloids of the Flora of Siberia and Altai. XXI.* 5′-(1,2,3-Triazolyl)-Substituted Lappaconitine Derivatives. Chem Nat Compd 51, 1142–1146 (2015). https://doi.org/10.1007/s10600-015-1511-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-015-1511-5