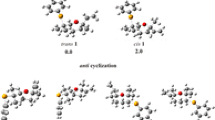
New 4a-quinopimaric acid derivatives were synthesized via a Diels–Alder reaction of levopimaric acid with 2-acetyl- or 2-(methoxycarbonyl)-1,4-benzoquinone and were characterized using elemental analysis and NMR spectroscopy. Thermodynamic and activation parameters of the Diels–Alder reactions of levopimaric acid and a model diene (7-isopropyl-1,2,3,4,4a,5-hexahydronaphthalene) with 2-acetyl- or 2-(methoxycarbonyl)-1,4-benzoquinone were calculated by density-functional theory in order to explain the observed regioselective cycloaddition.


Similar content being viewed by others
References
G. A. Tolstikov, T. G. Tolstikova, E. E. Shul'ts, S. E. Tolstikov, and M. V. Khvostov, Resinous Acids of Russian Conifers. Chemistry, Pharmacology [in Russian], Geo, Novosibirsk, 2011, 395 pp.
G. F. Vafina, R. R. Fazlyev, F. Z. Galin, and L. V. Spirikhin, Zh. Org. Khim., 45 (4), 515 (2009); G. A. Tolstikov, E. E. Shults, T. Sh. Mukhametyanova, I. P. Baikova, and L. V. Spirikhin, Zh. Org. Khim., 29, 698 (1993).
N. Ardabilchi, J. M. Bruce, and J. Khalafy, J. Sci. Islamic Repub. Iran, 11 (3), 195 (2000).
B. Kumar, S. M. Suryawanshi, and D. S. Bhakuni, Tetrahedron Lett., 36 (26), 4625 (1995).
A. Mukhopadhyay, S. M. Ali, M. Husain, S. N. Suryawanshi, and D. S. Bhakuni, Tetrahedron Lett., 30 (14), 1853 (1989).
G.-R. Cai, Z. Guan, and Y.-H. He, Synth. Commun., 41, 3016 (2011).
F. A. Carey and R. J. Sundberg, Advanced Organic Chemistry. Part B: Reaction and Synthesis, Kluwer Academic/Plenum Publishers, 2001, 965 pp.
M. F. Ansell, B. W. Nash, and D. A. Wilson, J. Chem. Soc., 3012 (1963).
N. N. Vorozhtsov and V. P. Mamaev, Sbornik Statei Obshch. Khim., 1, 533 (1953).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, and D. J. Fox, Gaussian 09, Revision C.01, Gaussian Inc., Wallingford, CT (2010).
A. D. Becke, J. Chem. Phys., 98, 5648 (1993).
C. Lee, W. Yang, and R. G. Parr, Phys. Rev. B, 37, 785 (1988).
B. Miehlich, A. Savin, H. Stoll, and H. Preuss, Chem. Phys. Lett., 157, 200 (1989).
A. D. McLean and G. S. Chandler, J. Chem. Phys., 72, 5639 (1980).
S. S. Borisevich, I. Tsipisheva, A. V. Kovalskaya, and S. L. Khursan, J. Theor. Comput. Chem., 13 (6), 1450048 (2014); DOI: 10.1142/S0219633614500485
J. Tomasi, B. Mennucci, and R. Cammi, Chem. Rev., 105, 2999 (2005)
Acknowledgment
The work was sponsored by a Grant of the RF President for Leading Scientific Schools No. NSh-1700.2014.3.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 6, November–December, 2015, pp. 964–969.
Rights and permissions
About this article
Cite this article
Vafina, G.F., Borisevich, S.S., Uzbekov, A.R. et al. Experimental and Theoretical Justification for the Regiospecific Cycloaddition of Levopimaric Acid to 2-Acetyl- or 2-(Methoxycarbonyl)-1,4-Benzoquinone. Chem Nat Compd 51, 1120–1125 (2015). https://doi.org/10.1007/s10600-015-1506-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-015-1506-2