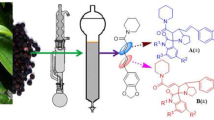
The stereochemistry of the 1,3-dipolar cycloaddition reaction of azomethine ylides to oxindolylidene derivatives of imidazo[4,5-e]-thiazolo[2,3-c][1,2,4]triazine leading to a mixture of diastereomeric derivatives of dispiro[imidazothiazolotriazine-7,3'-pyrrolidine-4',3''-oxindole] was studied. It was shown that the corresponding syn- and anti-stereoisomers can be isolated individually by fractional crystallization from reaction mixtures without the use of chromatographic methods.




Similar content being viewed by others
References
Jossang, A.; Jossang, P.; Hadi, H. A.; Sevenet, T.; Bodo, B. J. Org. Chem. 1991, 56, 6527.
Stuppner, H.; Sturm, S.; Konwalinka, G. Chromatographia 1992, 34, 597.
Cui, C.-B.; Kakeya, H.; Osada, H. Tetrahedron 1996, 52, 12651.
(a) Yu, B.; Yu, D.-Q.; Liu, H.-M. Eur. J. Med. Chem. 2015, 97, 673. (b) Zhao, Y.; Bernard, D.; Wang, S. BioDiscovery 2013, 8, e8950.
(a) Molteni, G.; Silvani, A. Eur. J. Org. Chem. 2021, 2021, 1653. (b) Zimnitskiy, N. S.; Barkov, A. Yu.; Kutyashev, I. B.; Korotaev, V. Yu.; Sosnovskikh, V. Ya. Chem. Heterocycl. Compd. 2021, 57, 743. (c) Izmest'ev, A. N.; Gazieva, G. A.; Kolotyrkina, N. G.; Daeva, E. D.; Kravchenko, A. N. Chem. Heterocycl. Compd. 2020, 56, 1569. (d) Izmest'ev, A. N.; Streltsov, A. A.; Karnoukhova, V. A.; Kolotyrkina, N. G.; Strelenko, Y. A.; Kravchenko, A. N.; Gazieva, G. A. ChemistrySelect 2022, 7, e202104128. (e) Klochkova, I. N.; Shchekina, M. P.; Anis'kov, A. A. Chem. Heterocycl. Compd. 2014, 50, 479. (f) Musabirov, I. Z.; Gataullin, R. R. Russ. J. Org. Chem. 2022, 58, 1369.
(a) Gugkaeva, Z. T.; Panova, M. V.; Smol'yakov, A. F.; Medvedev, M. G.; Tsaloev, A. T.; Godovikov, I. A.; Maleev, V. I.; Larionov, V. A. Adv. Synth. Catal. 2022, 364, 2395. (b) Filatov, A. S.; Knyazev, N. A.; Molchanov, A. P.; Panikorovsky, T. L.; Kostikov, R. R.; Larina, A. G.; Boitsov, V. M.; Stepakov, A. V. J. Org. Chem. 2017, 82, 959. (c) Knyazev, N. A.; Shmakov, S. V.; Pechkovskaya, S. A.; Filatov, A. S.; Stepakov, A. V.; Boitsov, V. M.; Filatova, N. A. Int. J. Mol. Sci. 2021, 22, 8264. (d) Kutyashev, I. B.; Ulitko, M. V.; Barkov, A. Yu.; Zimnitskiy, N. S.; Korotaev, V. Yu.; Sosnovskikh, V. Ya. Chem. Heterocycl. Compd. 2021, 57, 751.
(a) Izmest'ev, A. N.; Gazieva, G. A.; Karnoukhova, V. A.; Kravchenko, A. N. Org. Biomol. Chem. 2020, 18, 6905. (b) Izmest'ev, A. N.; Karnoukhova, V. A.; Larin, A. A.; Kravchenko, A. N.; Fershtat, L. L.; Gazieva, G. A. Int. J. Mol. Sci. 2022, 23, 13820.
(a) Izmest'ev, A. N.; Kravchenko, A. N.; Gazieva, G. A. Chem. Heterocycl. Compd. 2022, 58, 531. (b) Izmest'ev, A. N.; Anikina, L. V.; Zanin, I. E.; Kolotyrkina, N. G.; Izmalkova, E. S.; Kravchenko, A. N.; Gazieva, G. A. New J. Chem. 2022, 46, 11632. (c) Izmest'ev, A. N.; Gazieva, G. A.; Anikina, L. V.; Pukhov, S. A.; Karnoukhova, V. A.; Kolotyrkina, N. G.; Kravchenko, A. N. New J. Chem. 2021, 45, 12271.
(a) Shvets, A. A.; Kurbatov, S. V. Russ. Chem. Bull. 2010, 59, 1979. (b) Shvets, A. A.; Kurbatov, S. V. Chem. Heterocycl. Compd. 2012, 48, 799. (c) Gazieva, G. A.; Kolotyrkina, N. G.; Kravchenko, A. N.; Makhova, N. N. Russ. Chem. Bull. 2014, 63, 431.
Izmest'ev, A. N.; Kravchenko, A. N.; Gazieva, G. A. Mendeleev Commun. 2022, 32, 678.
CrysAlisPro. Version 1.171.41; Rigaku Oxford Diffraction, 2021.
Bruker. APEX-III; Bruker AXS, Inc.: Madison, 2019.
Krause, L.; Herbst-Irmer, R.; Sheldrick, G. M.; Stalke, D. J. Appl. Crystallogr. 2015, 48, 3.
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Adv. 2015, A71, 3.
Sheldrick, G. M. Acta Crystallogr., Sect. C: Struct. Chem. 2015, C71, 3.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2023, 59(8), 594–603
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Izmest’ev, A.N., Kravchenko, A.N. & Gazieva, G.A. A 1,3-dipolar cycloaddition of azomethine ylides to imidazo[4,5-e]thiazolo[2,3-c][1,2,4]triazine oxindolylidene derivatives in the synthesis of novel spirooxindole derivatives. Chem Heterocycl Comp 59, 594–603 (2023). https://doi.org/10.1007/s10593-023-03238-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-023-03238-3