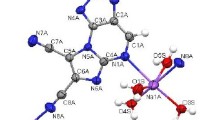
Cyclization of 1-hydrazinyl-3,3-dimethyl-3,4-tetrahydro-5H-benzo[c]azepine with ethyl orthoformate gave 5,5-dimethyl-6,7-dihydro-5Hbenzo[c]-1,2,4-triazolo[3,4-a]azepine, and its reaction with acetic acid afforded 3,5,5-trimethyl-9,10-dimethoxy-6,7-dihydro-5Н-benzo[c]-1,2,4-triazolo[3,4-a]azepine. Nitrosation of 1-hydrazino-3,3-dimethyl-3,4-tetrahydro-5H-benzo[c]azepine led to the formation of substituted 6,7-dihydro-5H-benzo[c]tetrazolo[5,1-a]azepines.




Similar content being viewed by others
References
Mashkovskii, M. D. Lekarstvennye sredstva (Drugs [in Russian]); Moscow: Novaya volna, 2019, 16th ed., p. 1216.
Varlamov, A. V.; Grudinin, D. G.; Gherhyshev, A. I.; Lenov, A. N.; Lobanov, I. Yu.; Borisov, R. S.; Zubkov, F. I. Russ. Chem. Bull. 2004, 53, 1711.
Glushkov, V. A.; Babentsev, D. N.; Dmitriev, M. V.; Stepanova, K. A.; Kharintseva, A. Yu.; Simakhina, A. E. Chem. Heterocycl. Compd. 2021, 57, 63.
Kasparek, S. Adv. Heterocycl. Chem. 1974, 17, 45.
Kouznetsov, V.; Palma, A.; Ewert, C. Curr. Org. Chem. 2001, 5, 519.
Nedolya, N. A.; Trofimov, B. A. Chem. Heterocycl. Compd. 2013, 49, 152.
Danyliuk, I. Yu.; Vas'kevich, R. I.; Vas'kevich, A. I.; Vovk, M. V. Chem. Heterocycl. Compd. 2019, 55, 802.
Velasco-Rubio, A.; Varela, J. A.; Saa, C. Adv. Synth. Catal. 2020, 362, 4861.
Basavaiah, D.; Satyanarayana, T. Chem. Commun. 2004, 32.
Li, X.; Zhou, G.; Du, X.; Wang, T.; Zhang, Z. Org. Lett. 2019, 21, 5630.
Lam, H.; Lautens, M. Synthesis 2020, 2427.
Sherikar, M. S.; Devarajappa, R.; Prabhu, K. R. J. Org. Chem. 2021, 86, 4625.
Bian, M.; Ma, K.; Mawjuda, H.; Yu, X.; Li, X.; Gao, H.; Zhou, Z.; Yi, W. Org. Biomol. Chem. 2019, 17, 6114.
Kurihara, T.; Kojima, M.; Yoshino, T.; Matsunaga, S. J. Am. Chem. Soc. 2022, 144, 7058.
Xu, Y.; Zhang, L.; Liu, M.; Zhang, X.; Zhang, X.; Fan. X. Org. Biomol. Chem. 2019, 17, 8706.
Krasniqi, B.; Dehaen, W. Org. Lett. 2019, 21, 5002.
Khomenko, D. M.; Doroshuk, R. O.; Ohorodnik, Yu. M.; Ivanova, H. V.; Zakharchenko, B. V.; Raspertova, I. V.; Vashenko, O. V.; Dobrydnev, A. V.; Grygorenko, O. O.; Lampeka, R. D. Chem. Heterocycl. Compd. 2022, 58, 116.
Verma, N.; Bera, S.; Mobdal, D. Chem. Heterocycl. Compd. 2022, 58, 73.
Alexandrov, B. B.; Glushkov, V. A.; Glushkova, E. N.; Gorbunov, A. A.; Shklyaev, V. S.; Shklyaev, Yu. V. Chem. Heterocycl. Compd. 1994, 30, 449.
Glushkov, V. A.; Shklyaev, Yu. V.; Maiorova, O. A.; Postanogova, G. A.; Feshina, E. V. Chem. Heterocycl. Compd. 2000, 36, 319.
Sokol, V. I.; Ryabov, M. A.; Merkur'eva, N. Yu.; Davydov, V. V.; Shklyaev, Yu. V.; Glushkov, V. A.; Zaitzev, B. E. Russ. Chem. Bull. 1995, 44, 2364.
CrysAlisPro, Version 1.171.37.33 (release 27-03-2014); Agilent Technologies.
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. J. Appl. Crystallogr. 2009, 42, 339.
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, A64, 112.
Sheldrick, G. M. Acta Crystallogr., Sect. C: Struct. Chem. 2015, C71, 3.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2022, 58(12), 727–731
Supplementary Information
ESM 1
(PDF 34662 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Glushkov, V.A., Mosheva, K.A., Zaitsev, M.M. et al. Synthesis of 6,7-dihydro-5H-benzo[c]-1,2,4-triazolo[3,4-a]azepines and 6,7-dihydro-5H-benzo[c]tetrazolo[5,1-a]azepines. Chem Heterocycl Comp 58, 727–731 (2022). https://doi.org/10.1007/s10593-023-03149-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-023-03149-3