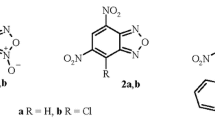
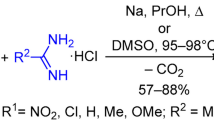
The use of substituted salicylaldehyde, a dicarbonyl compound, and a nitrogen base as starting compounds in the Hantzsch and Biginelli reactions leads to the formation of not only the expected reaction products, but also O-bridged derivatives isomeric to them. The review summarizes the results of studies of such Hantzsch and Biginelli reactions, as well as modified versions of these reactions with starting compounds, acid catalysts, and solvents of different nature. The routes of formation and chemical transformations of O-bridged structures, which represent the class of oxazocines and oxadiazocines with various substituents (H, Alk, Ar, Hal, OH, OAlk, NH2, etc.) and functional groups (Ac, CHO, CO2Alk, COCO2Alk, CONR2, CN, NO2), are discussed. Data on the biological activity of some functional derivatives are presented.





Similar content being viewed by others
References
(a) Lavilla, R. J. Chem. Soc., Perkin Trans. 1 2002, 1141. (b) Saini, A.; Kumar, S.; Sandhu, J. S. J. Sci. Industr. Res. 2008, 67, 95. (c) Wan, J.-P.; Liu, Y. RSC Adv. 2012, 2, 9763. (d) Triggle, D. K.; Langs, D. A.; Janis, R. A. Med. Res. Rev. 1989, 9, 123. (e) Singh, K.; Singh, K. Adv. Heterocycl. Chem. 2012, 105, 223. (f) Suresh; Sandhu, J. S. ARKIVOC 2012, (i), 66. f Sepehri, S.; Perez Sanchez, H.; Fassihi, A. J. Pharm. Pharm. Sci. 2015, 18, 1.
(a) Kappe, C. O. QSAR Comb. Sci. 2003, 22, 630. (b) Vdovina, S. V.; Mamedov, V. A. Russ. Chem. Rev. 2008, 77, 1017. (c) Heravi, M. M.; Asadi, S.; Lashkariani, B. M. Mol. Diversity 2013, 7, 389. (d) Heravi, M. M.; Moradi, R.; Mohammadkhani, L.; Moradi, B. Mol. Diversity 2018, 22, 751. (e) Gharui, Ch.; Pan, S. Ch. Org. Biomol. Chem. 2019, 17, 5190. (f) Chopda, L.; Dave, P. ChemSelect 2020, 5, 5552.
(a) Aron, Z. D.; Overman, L. E. Chem. Commun. 2004, 253. (b) Shkurko, O. P.; Tolstikova, T. G.; Sedova, V. F. Russ. Chem. Rev. 2016, 85, 1056. (c) Kaur, R.; Chaudhary, S.; Kumar, K.; Gupta, M. K.; Rawal, R. K. Eur. J. Med. Chem. 2017, 132, 108. (d) Naikoo, R. A.; Mir, M. A.; Bhat, S.; Tomar, R.; Bhat, R. A.; Malla, M. A. Curr. Bioact. Compd. 2016, 12, 236. (e) Santana Matos, L. H.; Teixeira Masson, F.; Simeoni, L. A.; Homem-de-Mello, M. Eur. J. Med. Chem. 2018, 143, 1779.
(a) Folkers, K.; Harwood, H. J.; Johnson, T. B. J. Amer. Chem. Soc. 1932, 54, 3751. (b) Ehsan, A.; Karimullah Pakistan J. Sci. Ind. Res. 1967, 10, 83; Chem. Abstr. 1968, 68, 78231.
Koelsch, C. F.; Freerks, M. C. J. Org. Chem. 1953, 18, 1538.
Světlík, J.; Tureček, F.; Hanuš, V. J. Chem. Soc., Perkin Trans. 1 1987, 563.
Kanematsu, K.; Parfitt, R. T.; Jacobson, A. E.; Ager, J. H.; May, E. L. J. Am. Chem. Soc. 1968, 90, 1064.
Murphy, J. G.; Ager, J. H.; May, E. L. J. Org. Chem. 1960, 25, 1386.
Boehm, Th.; Themlitz, R. Arch. Pharm. 1934, 272, 406.
Razdan, R. K.; Pars, G.; Zitko, B. A.; Kane, V. V.; Tompson, W. R. Tetrahedron Lett. 1973, 1623.
Biala, J.; Czarnocki, Z.; Maurin, J. K. Tetrahedron Asymm. 2002, 13, 1021.
Tkachenko, V. V.; Muravyova, E. A.; Desenko, S. M.; Shishkin, O. V.; Shishkina, S. V.; Sysoiev, D. O.; Müller, T. J. J.; Chebanov, V. A. Beilstein J. Org. Chem. 2014, 10, 3019.
Svetlik, J.; Hanuš, V.; Bella, J. J. Chem. Res., Synop. 1991, 4.
Světlík, J.; Tureček, F.; Hanuš, V. J. Chem. Soc., Perkin Trans. 1 1988, 2053.
Světlík, J.; Hamuš, V.; Bella, J. Liebigs Ann. Chem. 1989, 91.
Světlík, J.; Goljer, I.; Tureček, F. J. Chem. Soc., Perkin Trans. 1 1990, 1315.
Světlík, J.; Veizerová, L. Helv. Chim. Acta 2011, 94, 199.
Vachan, B. S.; Karuppasamy, M.; Jan, G.; Bhuvanesh, N.; Maheswari, C. U.; Sridharan, V. J. Org. Chem. 2020, 85, 8062.
(a) Claremon, D. A.; Hirshfield, J.; Lumma, P. K.; McClure, D. E.; Springer, J. P. Synthesis 1986, 144. (b) Claremon, D. A.; Young, S. D. Tetrahedron Lett. 1985, 26, 5417.
Kuckländer, U.; Ulmer, P.; Zerta, G. Arch. Pharm. 1989, 322, 437.
Liepin’sh, É. É.; Skrastin’sh, I. P.; Kastron, V. V.; Dubur, G. Ya. Chem. Heterocycl. Compd. 1989, 25, 1316.
Sahn, J. J.; Martin, S. F. Tetrahedron Lett. 2011, 52, 6855.
Girke, W. P. K. Chem. Ber. 1979, 112, 1.
Bartashevich, E.V.; Plekhanov, P. V.; Rusinov, G. L.; Potemkin, V. A.; Belik, A. V.; Chupakhin, O. N. Russ. Chem. Bull. 1999, 48, 1553.
Plekhanov, V. P. Extended Abstract of PhD (Chem.) Dissertation, Yekaterinburg, 2005.
Gazizov, A. S.: Kharitonova, N. I.; Syakaev, V. V.; Dobrynin, A. B.; Burilov, A. R.; Pudovik, M. A. Monatsh. Chem. 2016, 147, 2113.
Weis, A. L.; Frolow, F. J. Org. Chem. 1984, 49, 3635.
Rehani, R.; Shah, A. C.; Arya, V. P. Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem. 1994, 33B, 775.
Kurbanova, М. М. Russ. J. Org. Chem. 2010, 46, 599.
Sedova, V. F.; Krivopalov, V. P.; Shkurko, O. P. Chem. Heterocycl. Compd. 2017, 53, 1163.
Finkelstein, B. L.; Benner, E. A.; Hendrixson, M. C.; Kranis, K. T.; Rauh, J. J.; Sethuraman, M. R.; McCann, S. F. Bioorg. Med. Chem. 2002, 10, 599.
McCann, S. F.; Finkelstein, B. L. WO Patent 9946266; Chem. Abstr. 1999, 131, 214308.
Baldwin, J. J.; Claremon, D. A.; McClure, D. E. US Patent 4609494, 1986; Chem. Abstr. 1987, 106, 18636.
Velpuesta Fernandez, M.; Lopez Herrera, F. J.; Lupión Cobos, T. Heterocycles 1986, 24, 679.
Velpuesta Fernandez, M.; Lopez Herrera, F. J.; Lupión Cobos, T. Heterocycles 1988, 27, 2133.
Lopez Aparicio, F. J.; Lopez Sastre, J. A.; Molina Molina, J. Carbohydr. Res. 1981, 95, 113.
Abbas, E. M. H.; Abdallah, S. M.; Abdoh, M. H.; Tawfik, H. A.; El-Hamouly, W. S. Turk. J. Chem. 2008, 32, 297.
Matache, M.; Dobrota, C.; Bogdan, N. D.; Dumitru, I.; Ruta, L. L.; Paraschivescu, C. C.; Farcasanu, I. C.; Baciu, I.; Funeriu, D. P. Tetrahedron 2009, 65, 5949.
Cheng, Q.; Wang, Q.; Xu, X.; Ruan, M.; Yao, H.; Yang, X. J. Heterocycl. Chem. 2010, 47, 624.
Cheng, Q.; Wang, Q.; Tan, T.; Chen, N.; Shuai, M. J. Heterocycl. Chem. 2012, 49, 1352.
(a) Jing, X.; Li, Zh.; Pan, X.; Wang, Q.; Yan, Ch.; Zhu, H. Synth. Commun. 2009, 39, 3796. (b) Zhu, J.; Zhang, M.; Liu, B.; Li, X. Chem. Lett. 2009, 38, 56.
Liu, Q.; Xu, J.; Teng, F.; Chen, A.; Pan, N.; Zhang, W. J. Heterocycl. Chem. 2014, 51, 741.
Kulakov, I. V.; Ogurtsova, D. N.; Seilkhanov, T. M.; Gatilov, Yu. V.; Fisyuk, A. S. J. Heterocycl. Chem. 2018, 55, 923.
Jafari-Chermahini, M. T.; Tavakol, H. ChemistrySelect 2019, 4, 1895.
Zheng, R.; Wang, X.; Xu, H.; Du, J. Synth. Commun. 2006, 36, 1503.
Salehi, H.; Guo, Q.-X. Chin. J. Chem. 2005, 23, 91.
Mobinikhaledi, A.; Foroughifar, N.; Mosleh, T.; Jabbarpour, M. Chem. Sci. Trans. 2015, 4, 1066.
Světlík, J.; Veizerová, L.; Kettmann, V. Tetrahedron Lett. 2008, 49, 3520.
Mobinikhaledi, A.; Foroughifar, N.; Mosleh, T.; Hamta, A. Phosphorus, Sulfur Silicon Relat. Elem. 2012, 187, 728.
Kharaneko, O. I.; Pekhtereva, T. M.; Kharaneko, A. O. Russ. J. Org. Chem. 2019, 55, 1674.
El-Hamouly, W. S.; El-Khamry, A.-M. A.; Abbas, E. M. H. Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem. 2006, 45B, 2091.
El-Hamouly, W. S.; Tawfik, H. A.; Abbas, E. M. H. Green Chem. Lett. Rev. 2009, 2, 213.
Salehi, H.; Li, Q.-R.; Guo, Q.-X. Chin. J. Chem. Phys. 2006, 19, 84.
Zamaraeva, T. M.; Gein, V. L.; Buzmakova, N. A.; Dmitriev, M. V. Russ. J. Org. Chem. 2016, 52, 1022.
Gein, V. L.; Zamaraeva, T. M.; Buzmakova, N. A.; Dmitriev, M. V.; Nasakin, O. E.; Kazantseva, M. I. Russ. J. Org. Chem. 2017, 53, 86.
Gein, V. L.; Zamaraeva, T. M.; Dmitriev, M. V.; Nasakin, O. E. Russ. J. Org. Chem. 2017, 53, 869.
Gein, V. L.; Zamaraeva, T. M.; Buzmakova, N. A.; Rudakova, I. P.; Dmitriev, M. V. Pharm. Chem. J. 2018, 52, 515.
Sedova, V. F.; Krivopalov, V. P.; Gatilov, Yu. V.; Shkurko O. P. Mendeleev Commun. 2013, 23, 176.
Sedova, V. F.; Krivopalov, V. P.; Gatilov, Yu. V.; Shkurko, O. P. Russ. Chem. Bull. 2014, 63, 1378.
Sedova, V. F.; Krivopalov, V. P.; Shkurko, O. P. Russ. Chem. Bull. 2016, 65, 215.
Gümüs, M. K.; Gorobets, N. Yu.; Sedash, Yu. V.; Chebanov, V. A.; Desenko, S. M. Chem. Heterocycl. Compd. 2017, 53, 1261.
Gorobets, N. Yu.; Sedash, Yu. V.; Ostras, K. S.; Zaremba, O. V.; Shishkina, S. V.; Baumer, V. N.; Shishkin, O. V.; Kovalenko, S. M.; Desenko, S. M.; Van der Eycken, E. V. Tetrahedron Lett. 2010, 51, 2095.
Svetlik, J.; Veizerová, L.; Mayer, T. U.; Catarinella, M. Bioorg. Med. Chem. Lett. 2010, 20, 4073.
Alvim, H. G. O.; Da Silva Júnior, E. N.; Neto, B. A. D. RSC Adv. 2014, 4, 54282.
Shen, L.; Cao, S.; Wu, J.; Zhang, J.; Li, H.; Liu, N.; Qian, X. Green Chem. 2009, 11, 1414.
De Sousa, R. O. M. A.; da Penha, E. T.; Milagre, H. M. S.; Garden, S. J.; Esteves, P. M.; Eberlin, M. N.; Antunes, O. A. C. Chem.–Eur. J. 2009, 15, 9799.
Nagarajaiah, H.; Mukhopadhyay, A.; Moorthy, J. N. Tetrahedron Lett. 2016, 57, 5135.
Kolosov, M. A.; Orlov, V. D.; Beloborodov, D. A.; Dotsenko, V. V. Mol. Diversity 2009, 13, 5.
Kappe, C. O. J. Org. Chem. 1997, 62, 7201.
Ma, J. G.; Zhang, J. M.; Jiang, H. H.; Ma, W. Y.; Zhou, J. H. Chin. Chem. Lett. 2008, 19, 375.
Kappe, C. O.; Fabian, W. M. F.; Semones, M. A. Tetrahedron 1997, 53, 2803.
Baldwin, J. J.; Claremon, D. A.; Lumma, P. K.; Mc Clure, D. E.; Rosenthal, S. A.; Winquist, R. J.; Faison, E. P.; Kaczorowski, G. J.; Trumble, M. J.; Smith, G. M. J. Med. Chem. 1987, 30, 690.
Yar, M.; Bajda, M.; Shahzadi, L.; Shahzad, S. A.; Ahmed, M.; Ashraf, M.; Alam, U.; Khan, I. U.; Khan, A. F. Bioorg. Chem. 2014, 54, 96.
Bose, D. S.; Kumar, R. K.; Fatima, L. Synlett 2004, 279.
Bose, D. S.; Sudharshan, M.; Chavhan, S. W. ARKIVOC 2005, (iii), 228.
Fu, N.-Y.; Yuan, Y.-F.; Cao, Zh.; Wang, Sh.-W.; Wang, J.-T.; Peppe, C. Tetrahedron 2002, 58, 4801.
Fu, N.-Y.; Yuan, Y.-F.; Pang, M.-L.; Wang, J.-T.; Peppe, C. J. Organomet. Chem. 2003, 672, 52.
Boumoud, T.; Boumoud, B.; Rhouati, S.; Belfaitah, A.; Debache, A.; Mosset, P. Acta Chim. Slov. 2008, 55, 617.
Kumar, A.; Maurya, R. A. Tetrahedron Lett. 2007, 48, 4569.
Puripat, M.; Ramozzi, R.; Hatanaka, M.; Parasuk, W.; Parasuk, V.; Morokuma, K. J. Org. Chem. 2015, 80, 6959.
Raj, M. K.; Rao, H. S. P.; Manjunatha, S. G.; Sridharan, R.; Nambiar, S.; Keshwan, J.; Rappai, J.; Bhagat, S.; Shwetha, B. S.; Hegde, D.; Santhosh, U. Tetrahedron Lett. 2011, 52, 3605.
Kettmann, V.; Světlík, J.; Veizerová, L. Acta Crystallogr., Sect. E: Struct. Rep. Online 2010, E66, o1402.
Kettmann, V.; Světlík, J. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 1996, C52, 1496.
Cheng, Q.; Xu, X.; Zhang, H.; Ruan, M.; Lin, Q.; Yang, X. Acta Chim. Sinica. 2009, 67, 996.
Kurbanova, M. M.; Magerramov, A. M.; Novruzova, A. B.; Kurbanov, A. V.; Weng Ng, S. Acta Crystallogr., Sect. E: Struct. Rep. Online 2011, E67, o1156.
Kurbanova, M. M.; Kurbanov, A. V.; Novruzova, A. B.; Khrustalev, V. N.; Magerramov, A. M. J. Struct. Chem. 2010, 51, 998.
Kurbanova, M. M.; Kurbanov, A. V.; Askerov, R. K.; Allakhverdiev, M. A.; Khrustalev, V. N.; Magerramov, A. M. J. Struct. Chem. 2009, 50, 505.
Kulakov, I. V.; Talipov, S. A.; Shulgau, Z. T.; Seilkhanov, T. M. Chem. Heterocycl. Compd. 2014, 50, 1477.
Kettmann, V.; Světlík, J.; Veizerová, L. Acta Crystallogr., Sect. E: Struct. Rep. Online 2009, E65, o2967.
Kulakov, I. V.; Ogurtsova, D. N.; Shulgau, Z. T.; Seilkhanov, T. M.; Gatilov, Yu. V. Chem. Heterocycl. Compd. 2016, 52, 331.
Kettmann, V.; Svetlik, J. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 2000, C56, 1115.
Kettmann, V.; Svetlík, J. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 1997, C53, 1493.
Světlík, J.; Liptaj, T.; Tureček, F. J. Heterocycl. Chem. 1999, 36, 209.
Patra, G. C.; Bhunia, S. C.; Roy, M. K.; Pal, S. C. Synth. Commun. 2013, 43, 1646.
Svetlik J.; Veizerova, L.; Liptaj, T.; Kubista, J. ARKIVOC 2009, (x), 79.
Kim, D. H. J. Heterocycl. Chem. 1986, 23, 1471.
Stiasni, N.; Kappe, C. O. ARKIVOC 2002, (viii), 71.
Ren, Yu-W.; Wang, X.; Wang, W.; Li, B.; Shi, Z.-J.; Zhang, W. Tetrahedron Lett. 2011, 52, 192.
Remy, D. C.; King, S. W.; Cochran, D.; Springer, J. P.; Hirshfield, J. J. Org. Chem. 1985, 50, 4120.
(a) Claremon, D. A. US Patent 4552881; Chem. Abstr. 1986, 104, 186312. (b) Claremon, D. A. EU Patent 163238; Chem. Abstr. 1986, 104, 168500.
Svetlik, J.; Veverka, M. CZ Patent CS 272723; Chem. Abstr. 1992, 117, 212516.
Sallum, L. O.; Custodio, J. M. F.; Rodrigues, A. C. C.; Ribeiro, J. F. R.; Bezerra, B. P.; Ayala, A. P.; Ramos, L. M.; Camargo, A. J.; Napolitano, H. B. Z. Kristallogr. 2019, 234, 657.
Moon, R. T.; Bichele, T. L.; Camp, N. D.; Haggarty, S.; Fass, D. WO Patent 2010075282; Chem. Abstr. 2010, 153, 135836.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2022, 58(6/7), 279–300
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shkurko, O.P. Bridged 1,3(1,5)-benzoxazocines and 1,3,5-benzoxadiazocines as products of the Hantzsch and Biginelli reactions. Chem Heterocycl Comp 58, 279–300 (2022). https://doi.org/10.1007/s10593-022-03085-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-022-03085-8