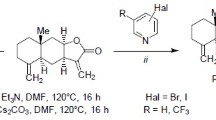
Modification of eudesmane-type lactones isoalantolactone and 4,15-epoxyisoalantolactone was carried out at the active methylene group. The reaction of isoalantolactone with hydrazoic acid, which was formed in situ from sodium azide and acetic acid in the presence of triethylamine in toluene or DMF, proceeds in a controllable manner and, depending on the nature of the solvent and reaction conditions, leads to (11R)-13-azido-11,13-dihydroisoalantolactone, the spirocyclic compound (11R)-aziridino[11,13]dihydroisoalantolactone, and the product of the selective opening of the aziridine ring with the azide anion (11S)-11-amino-13-azido-11,13-dihydroisoalantolactone. The reaction of 4,15-epoxyisoalantolactone with hydrazoic acid proceeds similarly to form the corresponding 13-azido-, (11R)-aziridino[11,13]-, or (11S)-11-amino-13-azido-4,15-epoxy-11,13-dihydroisoalantolactones. The regioselective synthesis of libraries of (11R)-13-(1H-1,2,3-triazolyl)-11,13-dihydroisoalantolactones and (11S)-11-amino-13-(1H-1,2,3-triazolyl)-11,13-dihydroisoalantolactones containing various aryl or hetaryl substituents at the C-4 position of the triazole ring was carried out by the CuAAC reaction of 13-azido-substituted 11,13-dihydroisoalantolactones with terminal acetylenes.

Similar content being viewed by others
References
(а) Santana, A.; Molinillo, J. M. G.; Macías, F. A. Eur. J. Org. Chem. 2015, 2093. a Ghantous, A.; Sinjab, A.; Herceg, Z.; Darwiche, N. Drug Discovery Today 2013, 18, 894. b Woods, J. R.; Mo, H.; Bieberich, A. A.; Alavanja, T.; Colby, D. A. MedChemComm 2013, 4, 27.
(а) Klochkov, S. G.; Afanas'eva, S. V.; Pushin, A. N. Chem. Nat. Compd. 2006, 42, 400. [Khim. Prirod. Soedin. 2006, 325.] (b) Huo, Y.; Shi, H.; Li, W.; Wang, M.; Li, X. J. Pharm. Biomed. Anal. 2010, 51, 942.
(a) Belovodskii, A. V.; Shults, E. E.; Shakirov, M. M.; Bagryanskaya, I. Yu.; Gatilov, Yu. V.; Tolstikov, G. A. Russ. J. Org. Chem. 2010, 46, 1719. [Zh. Org. Khim. 2010, 46, 1710.] (b) Belovodskii, A. V.; Shul'ts, E. E.; Shakirov, M. M.; Romanov, V. E.; Elmuradov, B. Zh.; Shakhidoyatov, Kh. M.; Tolstikov G. A. Chem. Nat. Compd. 2011, 46, 880. [Khim. Prirod. Soedin. 2010, 747.] (с) Shul'ts, E. E.; Belovodskii, A. V.; Shakirov, M. M.; Tolstikov, G. A. Russ. Chem. Bull., Int. Ed. 2012, 61, 1975 [Izv. Akad. Nauk, Ser. Khim. 2012, 1959.]
(a) Patrushev, S. S.; Shakirov, M. M.; Rybalova, T. V.; Shul'ts, E. E. Russ. J. Org. Chem. 2013, 49, 1783. [Zh. Org. Khim. 2013, 49, 1802.] (b) Patrushev, S. S.; Shakirov, M. M.; Shults, E. E. Chem. Heterocycl. Compd. 2016, 52, 165. [Khim. Geterotsikl. Soedin. 2016, 52, 165.] (с) Patrushev, S. S.; Rybalova, T. V.; Ivanov, I. D.; Vavilin, V. A.; Shults, E. E. Tetrahedron 2017, 73, 2717.
Reshetnikov, D. V.; Patrushev, S. S.; Shults, E. E. Chem. Nat. Compd. 2020, 56, 855. [Khim. Prirod. Soedin. 2020, 733.]
(а) Lone, S. H.; Bhat, K. A.; Majeed, R.; Hami, A.; Khuroo, M. A. Bioorg. Med. Chem. Lett. 2014, 24, 1047. a Zhang, Q.; Lu, Y.; Ding, Y.; Zhai, J.; Ji, Q.; Ma, W.; Yang, M.; Fan, H.; Long, J.; Tong, Z.; Shi, Y.; Jia, Y.; Han, B.; Zhang, W.; Qiu, C.; Ma, X.; Li, Q.; Shi, Q.; Zhang, H.; Li, D.; Zhang, J.; Lin, J.; Li, L.-Y.; Gao, Y.; Chen, Y. J. Med. Chem. 2012, 55, 8757. (с) Janganati, V.; Ponder, J.; Balasubramaniam, M.; Bhat-Nakshatri, P.; Bar, E. E.; Nakshatri, H.; Jordan, C. T.; Crooks, P. A. Eur. J. Med. Chem. 2018, 157, 562. b Zaki, M.; Oukhrib, A.; El Hakmaoui, A.; Hiebel, M.-A.; Berteina-Raboin, S.; Akssira, M. Z. Naturforsch., B: J. Chem. Sci. 2019, 74, 273.
Habib, F.; Alam, S.; Hussain, A.; Aneja, B.; Irfan, M.; Alajmi, M. F.; Hasan, P.; Khan, P.; Rehman, Md T.; Noman, O. M.; Azam, A.; Abid, M. J. Mol. Struct. 2020, 1201, 127144.
Zhang, H.; Zheng, X.; Li, J.; Liu, Q.; Huang, X.-X.; Ding, H.; Suzuki, R.; Muramatsu, M.; Song, S.-J. Eur. J. Med. Chem. 2021, 218, 113395.
(a) Nguyen, B. C. Q.; Takahashi, H.; Uto, Y.; Shahinozzaman, M. D.; Tawata, S.; Maruta, H. Eur. J. Med. Chem. 2017, 126, 270. (b) Xu, Z.; Zhao, S.-J.; Liu, Y. Eur. J. Med. Chem. 2019, 183, 111700. (с) Yang J.-J.; Yu, W.-W.; Hu, L.-L.; Liu, W.-J.; Lin, X.-H.; Wang, W.; Zhang, Q.; Wang, P.-L.; Tang, S-W.; Wang, X.; Liu, M.; Lu, W.; Zhang, H.-K. J. Med. Chem. 2020, 63, 569. c Ashour, H. F.; Abou-zeid, L. A.; El-Sayed, M. A.-A.; Selim, K. B. Eur. J. Med. Chem. 2020, 189, 112062.
(a) Shafi, S.; Alam, M. M.; Mulakayala, N.; Mulakayala, C.; Vanaja, G.; Kalle, A. M.; Pallu, R.; Alam, M. S. Eur. J. Med. Chem. 2012, 49, 324. (b) Haider, S.; Alam, M. S.; Hamid, H.; Shafi, S.; Nargotra, A.; Mahajan, P.; Nazreen, S.; Kalle, A. M.; Kharbanda, C.; Ali, Y.; Alam, A.; Panda, A. K. Eur. J. Med. Chem. 2013, 70, 579.
(a) Guerin, D. J.; Horstmann, T. E.; Miller, S. J. Org. Lett. 1999, 1, 1107. (b) Lakshmipathi, P.; Rama Rao, A. V. Tetrahedron Lett. 1997, 38, 2551. (c) Xu, L.-W.; Li, L.; Xia, C.-G.; Zhou, S.-L.; Li, J.-W. Tetrahedron Lett. 2004, 45, 1219.
(a) Kolb, H. C.; Finn, M. G.; Sharpless, K. B. Angew. Chem., Int. Ed. 2001, 40, 2004. (b) Tornøe, C. W.; Christensen, C.; Meldal, M. J. Org. Chem. 2002, 67, 3057.
Patrushev, S. S.; Shakirov, M. M.; Rybalova, T. V.; Shults, E. E. Chem. Heterocycl. Compd. 2014, 50, 1063. [Khim. Geterotsikl. Soedin. 2014, 1155.]
Creary, X.; Anderson, A.; Brophy, C.; Crowell, F.; Funk, Z. J. Org. Chem. 2012, 77, 8756.
Allen, F. H. Acta Crystallogr., Sect. B: Struct. Sci. 2002, B58, 380.
Cai, Y.-S.; Wu, Z.; Zheng, X.-Q.; Wang, C.; Wang, J.-R.; Zhang, X.-X.; Qiu, G.; Zhu, K.; Cao, Sh.; Yu, J. Org. Chem. Front. 2020, 7, 303.
Shi, Z.-R.; Shen, Yu.-H.; Zhang, X.-Yu.; Fang, X.; Zeng, R.-T.; Liu, Q.-X.; Zhuo, Zh.-G.; Feng, F.; Zhang, W.-D. RSC Adv. 2015, 5, 91640.
Klochkov, S. G.; Anan'ev, I. V.; Pukhov, S. A.; Afanas'eva, S. V. Chem. Heterocycl. Compd. 2012, 48, 698. [Khim. Geterotsikl. Soedin. 2012, 750.]
Cai, Y.-S.; Wu, Z.; Wang, J.-R.; Zheng, X.-Q.; Xu, J.; Qiu, G.; Yu, J. Org. Lett. 2019, 21, 9478.
Rowland, R. S.; Taylor, R. J. Phys. Chem. 1996, 100, 7384.
Srivastava, S. C.; Mehra, M. M.; Trivedi, G. K.; Bhattacharyya, S. C. Indian J. Chem. 1971, 9, 512.
Kulyyasov, A. T.; Seitembetov, T. S.; Turdybekov, K. M.; Adekenov, S. M. Chem. Nat. Compd. 1996, 32, 869. [Khim. Prirod. Soedin. 1996, 879.]
Aly, R. E. S.; Saad, H. A.; Mohamed, M. A. M. Bioorg. Med. Chem. Lett. 2015, 25, 2824.
Sheldrick, G. M. SADABS, Program for Area Detector Adsorption Correction; Institute for Inorganic Chemistry, University Gӧttingen, 1996.
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, A64, 112.
Spek, A. L. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2009, D65, 148.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2021, 57(11), 1116–1129
Supplementary Information
ESM 1
(PDF 3387 kb)
Rights and permissions
About this article
Cite this article
Patrushev, S.S., Rybalova, T.V. & Shults, E.E. Synthetic transformations of sesquiterpene lactones. Controllable synthesis of 11,13-dihydroisoalantolactone azides and 13-(1,2,3-triazolyl)eudesmanolides based on sesquiterpene lactones. Chem Heterocycl Comp 57, 1116–1129 (2021). https://doi.org/10.1007/s10593-021-03030-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-021-03030-1