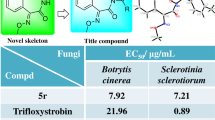
A series of 6H-benzimidazo[1,2-c][1,3]benzoxazin-6-one derivatives were synthesized in moderate to good yield by reaction of 2-(1H-benzimidazol-2-yl)phenols with triphosgene, and the structures of the target compounds were characterized by NMR, IR, and HRMS methods. The fungicidal activity of the compounds was evaluated at 50 μg/ml concentration, and unsubstituted 6H-benzimidazo-[1,2-c][1,3]benzoxazin-6-one showed 75.1% activity against Sclerotinia sclerotium, which was higher than that of chlorothalonil.

Similar content being viewed by others
References
Waisser, K.; Petrlíkova, E.; Peřina, M.; Klimešova, V.; Kuneš, J.; Palát, K., Jr.; Kaustová, J.; Dahse, H. M.; Möllmann, U. Eur. J. Med. Chem. 2010, 45, 2719.
Velappan, A. B.; Kesamsetty, D.; Datta, D.; Ma, R.; Hari, N.; Franzblau, S. G.; Debnath, J. Eur. J. Med. Chem. 2020, 208, 112835.
Varshney H.; Ahmad, A.; Rauf, A.; Husain, F. M.; Ahmad, I. J. Saudi Chem. Soc. 2017, 21, S394.
Madhavan, G. R.; Chakrabarti, R.; Reddy, K. A.; Rajesh, B. M.; Balraju, V.; Rao, P. B.; Rajagopalan, R.; Iqbal, J. Bioorg. Med. Chem. 2006, 14, 584.
Ihmaid, S.; Ahmed, H. E. A.; Al-Sheikh Ali, A.; Sherif, Y. E.; Tarazi, H. M.; Riyadh, S. M.; Zayed, M. F.; Abulkhair, H. S.; Rateb, H. S. Bioorg. Chem. 2017, 72, 234.
Morrison R.; Al-Rawi, J. M. A.; Jennings, I. G.; Thompson, P. E.; Angove, M. J. Eur J. Med. Chem. 2016, 110, 326.
Jiang, S.; Awadasseid, A.; Narva, S.; Cao S; Tanaka, Y.; Wu, Y.; Wei, F.; Zhao, X.; Wei, C.; Zhang, W. Life Sci. 2020, 258,118252.
Bari, A.; Khan, Z. A.; Shahzad, S. A.; Naqvi, S. A. R.; Khan, S. A.; Amjad, H.; Iqbal, A.; Yar, M. J. Mol. Struct. 2020, 1214, 128252.
Popov, L. D.; Shcherbakov, I. N.; Bulanov, A. O.; Kobeleva, O. I.; Valova, T. M.; Barachevsky, V. A. Russ. Chem. Bull., Int. Ed 2009, 58, 2418. [Izv. Akad. Nauk, Ser. Khim. 2009, 2340.]
Ozhogin, I. V.; Tkachev, V. V.; Lukyanov, B. S.; Ozhogin, I. V.; Tkachev, V. V.; Lukyanov, B. S.; Mukhanov, E. L.; Rostovtseva, I. A.; Lukyanova, M. B.; Shilov, G. V.; Strekal, N. D.; Aldoshin, S. M.; Minkin, V. I. J. Mol. Struct. 2018, 1161, 18.
Mayence, A.; Vanden Eynde, J. J.; Kaiser, M.; Brun, R.; Yarlett, N.; Huang, T. L. Bioorg. Med. Chem. 2011, 19, 7493.
Bandyopadhyay, P.; Sathe, M.; Tikar, S. N.; Yadav, R.; Sharma, P.; Kumar, A.; Kaushik, M. P. Bioorg. Med. Chem. Lett. 2014, 24, 2934.
Abdel-Motaal, M.; Almohawes, K.; Tantawy, M. A. Bioorg. Chem. 2020, 101, 103972.
Li, Y.; Zhou, X.; Wu, H.; Yu, Z.; Li, H.; Yang, S. Ind. Crops Prod. 2020, 150, 112406.
Meshram, G. A.; Vala, V. A. Chem. Heterocycl. Compd. 2015, 51, 44. [Khim. Geterotsikl. Soedin. 2015, 51, 44.]
Bocion, P. F.; Cattanach, C. J.; Eggenberg, P.; Gressel, J.; Hagmann, M. L.; Malkin, S.; Wenger, J. Pestic. Biochem. Physiol. 1987, 28, 75.
Akhtar, W.; Khan, M. F.; Verma, G.; Shaquiquzzaman, M.; Rizvi, M. A.; Mehdi, S. H.; Akhter, M.; Alam, M. M. Eur. J. Med. Chem. 2017, 126, 705.
Jornet, D.; Castillo, M. A.; Sabater, M. C.; Tormos, R.; Miranda, M. A. J. Photochem. Photobiol., A 2013, 256, 36.
Feng, J.; Hu, Y.; Grant, E.; Lu, X. Food Chem. 2018, 239, 816.
Tang, Z.; Chen, W.; Zhu, Z.; Liu, H. Synth. Commun. 2012, 42, 1372.
Tang, Z.; Chen, W.; Zhu, Z.; Liu, H. J. Heterocycl. Chem. 2011, 48, 255.
Tang, Z.; Zhu, Z.; Xia, Z.; Liu, H.; Chen, J.; Xiao, W.; Ou, X. Molecules 2012, 17, 8174.
Tang, Z.; Li, X.; Yao, Y.; Qi, Y.; Wang, M.; Dai, N.; Wen, Y.; Wan, Y.; Peng, L. Bioorg. Med. Chem. 2019, 27, 2572.
Cui, L. J.; Xiao, S.-Y.; Yang, H.-S.; Xu, P.; Cheng, F. S.; Li, Z. H.; Liang, R. H.; Xia, Z. N. Chin. J. Org. Chem. 2011, 31, 672.
Dou, G.-L.; Sun, F.; Shi, D.-Q. Tetrahedron 2012, 68, 4852.
Tang, Z. L.; Ma, C. X.; Peng, L. F.; Wan, Y. C.; Li, Y. CN Patent 110218217.
This work was supported by the National Natural Science Foundation of China (No. 21877034, 21372070) and the Scientific Research Fund of Hunan Provincial Education Department (No. 17A066).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2021, 57(5), 581–587
Supplementary Information
ESM 1
(PDF 4891 kb)
Rights and permissions
About this article
Cite this article
Tan, Y., Tang, Z., Ma, C. et al. Synthesis and fungicidal activity of novel 6H-benzimidazo[1,2-c][1,3]benzoxazin-6-ones. Chem Heterocycl Comp 57, 581–587 (2021). https://doi.org/10.1007/s10593-021-02946-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-021-02946-y