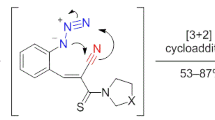
N-Boc-5-formylpyrazol-4-amines reacted with 1,3-cyclohexanediones in AcOH–1,4-dioxane medium in the presence of pyrrolidine, resulting in the formation of pyrazolo[4,3-b]quinolin-8-one derivatives that were converted into the respective oximes. A reaction of the latter with trifluoromethanesulfonic anhydride under mild conditions was used to synthesize hexahydropyrazolo[3',4':5,6]pyrido[3,2-b]-azepin-4-ium trifluoromethanesulfonates.


Similar content being viewed by others
References
Sher, P.; Wu, G.; Stouch, T.; Ellsworth, B. US Patent 20040002495.
Patel, S. WO Patent 2018073193.
Tung, J. S.; Garofalo, A. W.; Pleiss, M. A.; Wu, J.; Wone, D. W. G.; Guinn, A. C.; Dressen, D. B.; Neitz, R. J.; Marugg, J.; Neitzel, M. WO Patent 2004098589.
Fisher, C.; Zultanski, S. L.; Zhou, H.; Methot, J. L.; Shah, S.; Hayashi, I.; Hughes, B. L.; Moxham, C. M.; Bays, N. W.; Smotrov, N.; Hill, A. D.; Pan, B.-S.; Wu, Z.; Moy, L. Y.; Tanga, F.; Kenific, C.; Cruz, J. C.; Walker, D.; Bouthillette, M.; Nikov, G. N.; Deshmukh, S. V.; Jeliazkova-Mecheva, V. V.; Diaz, D.; Michener, M.; Cook, J. J.; Munoz, B.; Shearman, M. S. Bioorg. Med. Chem. Lett. 2015, 25, 3488.
Stukenbrock, H.; Mussmann, R.; Geese, M.; Ferandin, Y.; Lozach, O.; Lemcke, T.; Kegel, S.; Lomow, A.; Burk, U.; Dohrmann, C.; Meijer, L.; Austen, M.; Kunick, C. J. Med. Chem. 2008, 51, 2196.
Kunick, C.; Lauenroth, K.; Leost, M.; Meijer, L.; Lemske, T. Bioorg. Med. Chem. Lett. 2004, 14, 413.
Mussmann, R.; Kunick, C.; Stukenbrock, H.; Geese, M.; Kegel, S.; Burk, U. WO Patent 2006117221.
Egert-Schmidt, A.-M.; Dreher, J.; Dunkel, U.; Kohfeld, S.; Preu, L.; Weber, H.; Ehlert, J. E.; Mutschler, B.; Totzke, F.; Schächtele, C.; Kubbutat, M. H. G.; Baumann, K.; Kunick, C. J. Med. Chem. 2010, 53, 2433.
Abd El-Aal, H. A. K.; El-Emary, T. I. Austr. J. Chem. 2019, 72, 945.
Song, Y.-H.; Joc, B. S.; Lee, H. M. Heterocycl. Commun. 2009, 15, 203.
Gatta, F.; Pomponi, M.; Marta, M. J. Heterocycl. Chem. 1991, 28, 1301.
Yakovenko, G. G.; Lukianov, O. A.; Bol'but, A. V.; Vovk, M. V. Chem. Heterocycl. Compd. 2019, 55, 379. [Khim. Geterotsikl. Soedin. 2019, 55, 379.]
Yakovenko, G. G.; Lukianov, O. A.; Bol’but, A. V.; Vovk, M. V. Chem. Heterocycl. Compd. 2019, 55, 1211. [Khim. Geterotsikl. Soedin. 2019, 55, 1211.]
Yakovenko, G. G.; Lukianov, O. A.; Yagodkina-Yakovenko, M. S.; Bol’but, A. V.; Vovk, M. V. Chem. Heterocycl. Compd. 2020, 56, 347. [Khim. Geterotsikl. Soedin. 2020, 56, 347.]
Maquestiau, A.; Van Haverbeke. Y.; Vanden Eynde J.-J.; De Pauw, N. Bull. Soc. Chim. Belg. 1980, 89, 45.
Tolkunov, S. V.; Khyzhan, A. I.; Dulenko, V. I. Chem. Heterocycl. Compd. 2003, 39, 1627. [Khim. Geterotsikl. Soedin. 2003, 1849.]
Jössang-Yanagida, A.; Gansser, C. J. Heterocycl. Chem. 1978, 15, 249.
Muylaert, K.; Jatczak, M.; Wuyts, B.; De Coen, L. M.; Van Hecke, K.; Loones, H.; Keemink, J.; García, D.; Mangelickx, S.; Annaert, P.; Stevens, C. V. Synlett 2014, 1443.
Deng, Y.; Achab, A.; Becker, B. A.; Bharathan, I.; Fradera, X.; Gibeau, C.; Han, Y.; Li, D.; Liu, K.; Pu, Q.; Yu, W.; Zhang, H.; Bennett, J. D.; Sanyal, S.; Sloman, D. WO Patent 2019089412.
Pauton, M.; Aubert, C.; Blent, G.; Gruss-Leleu, F.; Roy, S.; Perrio, C. Org. Process Res. Dev. 2019, 23, 900.
Montavon, T. J.; Türkmen, Y. E.; Shamsi, N. A.; Miller, C.; Sumaria, C. S.; Rawal, V. H.; Kozmin, S. A. Angew. Chem., Int. Ed. 2013, 52, 13576.
Henrich, M.; Abel, U.; Muller, S.; Kubas, H.; Meyer, U.; Hechenberger, M.; Kauss, V.; Zemribo, R. WO Patent 2012052451.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2021, 57(2), 199–206
Rights and permissions
About this article
Cite this article
Yakovenko, G.G., Yagodkina-Yakovenko, M.S., Suykov, S.Y. et al. A Beckmann rearrangement initiated by trifluoromethanesulfonic anhydride in the synthesis of compounds containing a new pyrazolo[3',4':5,6]pyrido[3,2-b]azepine heterocyclic system. Chem Heterocycl Comp 57, 199–206 (2021). https://doi.org/10.1007/s10593-021-02893-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-021-02893-8