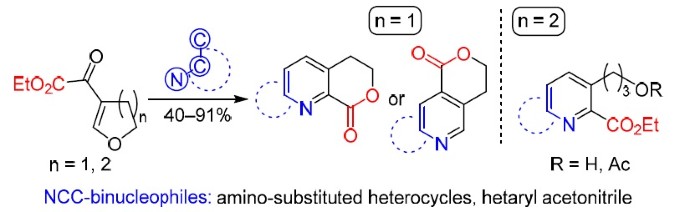
Reactions of five- and six-membered cyclic β-alkoxyvinyl α-keto esters and NCC-binucleophiles are described. The following binucleophiles were studied: heteroaromatic amines (pyrazoles, isoxazole, uracils, and isoquinolinone) and 2-(benzimidazolyl)acetonitrile. It was found that condensation proceeded regioselectively in the case of five-membered cyclic enone. Fused α-pyridine carboxylates, likely formed in situ, underwent lactonization, which resulted in six-membered lactones in moderate to excellent yields (53–91%). Only in the case of 3-unsubstituted 5-aminopyrazole, formation of γ-pyridine carboxylate followed by cyclization to isomeric lactone was observed. On the contrary, six-membered cyclic enone was reactive only toward heterocyclic amines under optimized conditions and provided open-chain α-pyridine carboxylates in 40–80% yield.




Similar content being viewed by others
References
(a) Mousseau, J. J.; Bull, J. A.; Ladd, C. L.; Fortier, A.; Sustac Roman, D.; Charette, A. B. J. Org. Chem.2011, 76, 8243. (b) Gunasekaran, P.; Indumathi, S.; Perumal, S. RSC Adv.2013, 3, 8318. (c) Vitaku, E.; Smith, D. T.; Njardarson, J. T. J. Med. Chem.2014, 57, 10257.
(a) Shamroukh, A. H.; Rashad, A. E.; Sayed, H. H. Phosphorus, Sulfur Silicon Relat. Elem.2005, 180, 2347. (b) Lominac, W. J.; D’Angelo, M. L.; Smith, M. D.; Ollison, D. A.; Hanna, J. M., Jr. Tetrahedron Lett.2012, 53, 906. (c) Lin, R.; Connolly, P. J.; Lu, Y.; Chiu, G.; Li, S.; Yu, Y.; Huang, S.; Li, X.; Emanuel, S. L.; Middleton, S. A.; Gruninger, R. H.; Adams, M.; Fuentes-Pesquera, A. R.; Greenberger, L. M. Bioorg. Med. Chem. Lett.2007, 17, 4297.
(a) Greenwood, D. Antimicrobial Drugs: Chronicle of a Twentieth Century Medical Triumph; Oxford University Press: Oxford, 2008. (b) Lingham, A. R.; Hawley, J. A.; Greaves, T.; Jackson, N.; Antolasic, F.; Hügel, H. M. Polyhedron2016, 120, 175. (c) Roecker, A. J.; Reger, T. S.; Mattern, M. C.; Mercer, S. P.; Bergman, J. M.; Schreier, J. D.; Cube, R. V.; Cox, C. D.; Li, D.; Lemaire, W.; Bruno, J. G.; Harrell, C. M.; Garson, S. L.; Gotter, A. L.; Fox, S. V.; Stevens, J.; Tannenbaum, P. L.; Prueksaritanont, T.; Cabalu, T. D.; Cui, D.; Stellabott, J.; Hartman, G. D.; Young, S. D.; Winrow, C. J.; Renger, J. J.; Coleman, P. J. Bioorg. Med. Chem. Lett.2014, 24, 4884. (d) Su, D.-S.; Lim, J. J.; Tinney, E.; Wan, B.-L.; Young, M. B.; Anderson, K. D.; Rudd, D.; Munshi, V.; Bahnck, C.; Felock, P. J.; Lu, M.; Lai, M.-T.; Touch, S.; Moyer, G.; DiStefano, D. J.; Flynn, J. A.; Liang, Y.; Sanchez, R.; Perlow-Poehnelt, R.; Miller, M.; Vacca, J. P.; Williams, T. M.; Anthony, N. J. J. Med. Chem.2009, 52, 7163.
(a) Gunasekaran, P.; Prasanna, P.; Perumal, S. Tetrahedron Lett.2014, 55, 329. (b) Rubin, L. J.; Galié, N.; Grimminger, F.; Grünig, E.; Humbert, M.; Jing, Z. C.; Keogh, A.; Langleben, D.; Fritsch, A.; Menezes, F.; Davie, N.; Ghofrani, H. A. Eur. Respir. J.2015, 45, 1303. (c) Khaybullina, D.; Patel, A.; Zerilli, T. Pharm. Ther.2014, 39, 749. (d) Halank, M.; Tausche, K.; Grünig, E.; Ewert, R.; Preston, I. R. Ther. Adv. Respir. Dis.2019, 13, 1. e Huang, S.; Lin, R.; Yu, Y.; Lu, Y.; Connolly, P. J.; Chiu, G.; Li, S.; Emanuel, S. L.; Middleton, S. A. Bioorg. Med. Chem. Lett.2007, 17, 1243. f Höhn, H.; Denzel, T.; Janssen, W. J. Heterocycl. Chem.1972, 9, 235. g Denzel, T.; Höhn, H. Arch. Pharm.1976, 309, 486. h Misra, R. N.; Rawlins, D. B.; Xiao, H.-Y.; Shan, W.; Bursuker, I.; Kellar, K. A.; Mulheron, J. G.; Sack, J. S.; Tokarski, J. S.; Kimball, S. D.; Webster, K. R. Bioorg. Med. Chem. Lett.2003, 13, 1133. (i) Fang, W. K.; Wang, L.; Corpuz, E. G.; Chow, K.; Im, W. B. WO Patent 2011041287A1.
Yoshimi, K.; Kozuka, M.; Sakai, J.; Iizawa, T. Shimizu, Y.; Kaneko, I.; Kojima, K.; Iwata, N. Jpn. J. Pharmacol.2002, 88, 174.
Edupuganti, R.; Wang, Q.; Tavares, C. D. J.; Chitjian, C. A.; Bachman, J. L.; Ren, P.; Anslyn, E. V.; Dalby, K. N. Bioorg. Med. Chem.2014, 22, 4910.
Lovering, F.; Bikker, J.; Humblet, C. J. Med. Chem.2009, 52, 6752.
(a) Ghaedi, A.; Bardajee, G. R.; Mirshokrayi, A.; Mahdavi, M.; Shafiee, A.; Akbarzadeh, T. RSC Adv.2015, 5, 89652. (b) Hamama, W. S.; Ibrahim, M. E.; Zoorob, H. H. Arch. Pharm.2012, 345, 468. (c) Morozova, A. D.; Muravyova, E. A.; Desenko, S. M.; Musatov, V. I.; Yedamenko, D. V.; Chebanov, V. A. Chem. Heterocycl. Compd.2016, 52, 934. [Khim. Geterotsikl. Soedin.2016, 52, 934.] (d) El-Borai, M. A.; Rizk, H. F.; Abd-Aal, M. F.; El-Deeb, I. Y. Eur. J. Med. Chem.2012, 48, 92. d Chebanov, V. A.; Sakhno, Y. I.; Desenko, S. M.; Chernenko, V. N.; Musatov, V. I.; Shishkina, S. V.; Shishkin, O. V.; Kappe, C. O. Tetrahedron2007, 63, 1229. e Maqbool, T.; Nazeer, A.; Khan, M. N.; Elliott, M. C.; Khan, M. A.; Ashraf, M.; Nasrullah, M.; Arshad, S.; Munawar, M. A. Asian J. Chem.2014, 26, 2870. f Dias, L. R. S.; Santos, M. B.; de Albuquerque, S.; Castro, H. C.; de Souza, A. M. T.; Freitas, A. C. C.; DiVaio, M. A. V.; Cabral, L. M.; Rodrigues, C. R. Bioorg. Med. Chem.2007, 15, 211. g Ramzan, A.; Siddiqui, S.; Irfan, A.; Al-Sehemi, A. G.; Ahmad, A.; Verpoort, F.; Chughtai, A. H.; Khan, M. A.; Munawar, M. A.; Basra, M. A. R. Med. Chem. Res.2018, 27, 388. h Becerra-Ruiz, M.; Vargas, V.; Jara, P. G.; Tirapegui, C.; Carrasco, C.; Núñez, M. T.; Lezana, N.; Galdámez, A.; Vilches-Herrera, M. Eur. J. Org. Chem.2018, 4795. i Dubovtsev, A. Yu.; Dmitriev, M. V.; Silaichev, P. S.; Antonov, D. I.; Maslivets, А. N. Synthesis2017, 2223. j Silaichev, P. S.; Bubnov, N. V.; Filimonov, V. O.; Denislamova, E. S.; Maslivets, A. N. Russ. J. Org. Chem.2013, 49, 1248. [Zh. Org. Khim.2013, 49, 1260.]
(a) Hughes, D. D.; Bagley, M. C. Synlett2002, 1332. (b) Mkrtchyan, S.; Iaroshenko, V. O.; Dudkin, S.; Gevorgyan, A.; Vilches-Herrera, M.; Ghazaryan, G.; Volochnyuk, D. M.; Ostrovskyi, D.; Ahmed, Z.; Villinger, A.; Sosnovskikh, V. YA.; Langer, P. Org. Biomol. Chem.2010, 8, 5280. (c) Giardina, G. A. M.; Artico, M.; Cavagnera, S.; Cerri, A.; Consolandi, E.; Gagliardi, S.; Graziani, D.; Grugni, M.; Hay, D. W. P.; Luttmann, M. A.; Mena, R.; Raveglia, L. F.; Rigolio, R.; Sarau, H. M.; Schmidt, D. B.; Zanoni, G.; Farina, C. Farmaco1999, 54, 364. (d) Sarmah, M. M.; Bhuyan, D.; Prajapati, D. RSC Adv.2015, 5, 12506. (e) Walsh, E. B.; Zhu, N.; Fang, G.; Wamhoff, H. Tetrahedron Lett.1988, 29, 4401. (f) Roh, Y. H.; Bae, J. W.; Nam, G. S. Kim, J. H.; Kim, S. H.; Yoon, C. M.; Synth. Commun.2000, 30, 81. (g) Rho, K. Y.; Kim, J. H.; Kim, S. H.; Yoon, C. M. Heterocycles1998, 48, 2521.
Goli-Garmroodi, F.; Omidi, M.; Saeedi, M.; Sarrafzadeh, F.; Rafinejad, A.; Mahdavi, M.; Bardajee, G. R.; Akbarzadeh, T.; Firoozpour, L.; Shafiee, A.; Foroumadi, A. Tetrahedron Lett.2015, 56, 743.
Blakemore, D. C.; Castro, L.; Churcher, I.; Rees, D. C.; Thomas, A. W.; Wilson, D. M.; Wood, A. Nat. Chem.2018, 10, 383.
(a) Chalyk, B. A.; Hrebeniuk, K. V.; Fil, Y. V.; Gavrilenko, K. S.; Rozhenko, A. B.; Vashchenko, B. V.; Borysov, O. V.; Biitseva, A. V.; Lebed, P. S.; Bakanovych, I.; Moroz, Y. S.; Grygorenko, O. O. J. Org. Chem.2019, 84, 15877. (b) Demchuk, O. P.; Hryshchuk, O. V.; Vashchenko, B. V.; Radchenko, D. S.; Kovtunenko, V. O.; Komarov, I. V.; Grygorenko, O. O. Eur. J. Org. Chem.2019, 5937. (c) Subota, A. I.; Lutsenko, A. O.; Vashchenko, B. V.; Volochnyuk, D. M.; Levchenko, V.; Dmytriv, Yu. V.; Rusanov, E. B.; Gorlova, A. O.; Ryabukhin, S. V.; Grygorenko, O. O. Eur. J. Org. Chem.2019, 3636. (d) Stepaniuk, O. O.; Rudenko, T. V.; Vashchenko, B. V.; Matvienko, V. O.; Kondratov, I. S.; Tolmachev, A. A.; Grygorenko, O. O. Tetrahedron2019, 75, 3472.
(a) Goryaeva, M. V.; Burgart, Y. V.; Ezhikova, M. A.; Kodess, M. I.; Saloutin, V. I. Beilstein J. Org. Chem.2015, 11, 385. (b) Bugera, M. Ya.; Tarasenko, K. V.; Kondratov, I. S.; Gerus, I. I.; Vashchenko, B. V.; Ivasyshyn, V. E.; Grygorenko, O. O. Eur. J. Org. Chem.2020, 1069. (c) Stepaniuk, O. O.; Rudenko, T. V.; Vashchenko, B. V.; Matvienko, V. O.; Kondratov, I. S.; Tolmachev, A. A.; Grygorenko, O. O. Synthesis2020. https://doi.org/10.1055/s-0039-1707987.
Stepaniuk, O. O.; Matvienko, V. O.; Kondratov, I. S.; Shishkin, O. V.; Volochnyuk, D. M.; Mykhailiuk, P. K.; Tolmachev, A. A. Synthesis2012, 895.
Armarego, W. L. F.; Chai, C. Purification of Laboratory Chemicals; Elsevier: Oxford, 2003.
Sheldrick, G. M. SHELX97. Programs for Crystal Structure Analysis (release 97-2); Institüt für Anorganische Chemie der Universität Göttingen, 1998.
Sheldrick, G. M. Acta Crystallogr., Sect. C: Struct. Chem.2015, C71, 3.
The authors acknowledge Enamine Ltd. for financial support. Oleksandr O. Grygorenko was also financially supported by the Ministry of Education and Science of Ukraine (grant No. 19БФ037-03).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2020, 56(3), 377–385
Electronic supplementary material
ESM 1
(PDF 5693 kb)
Rights and permissions
About this article
Cite this article
Stepaniuk, O.O., Vashchenko, B.V., Matvienko, V.O. et al. Reactions of cyclic β-alkoxyvinyl α-keto esters with heteroaromatic NCC-binucleophiles. Chem Heterocycl Comp 56, 377–385 (2020). https://doi.org/10.1007/s10593-020-02670-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-020-02670-z



