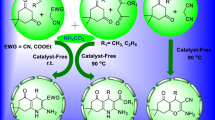
A facile, convenient, and efficient synthesis of new chromeno[4,3-b]quinoline and chromeno[4,3-b]pyridine derivatives has been accomplished via one-pot three-component reaction of 4-aminocoumarin, 1,3-dicarbonyl compounds (dimedone and Meldrum's acid), and substituted aromatic aldehydes in H2O or EtOH under reflux conditions using 10 mol % of guanidine hydrochloride as organocatalyst. Formation of products proceeds through tandem Knoevenagel and Michael reactions, followed by concomitant cyclization and dehydration or decarboxylation. A wide range of aromatic aldehydes were tolerated by the developed protocol. The advantages of this environmentally friendly synthetic approach are short reaction times, high atom economy, operational simplicity, application of readily available catalyst, z high yields of products, simple workup procedure, and possibility to purify products by recrystallization.
Similar content being viewed by others
References
Angelova, V. T.; Andreeva-Gateva, P. A.; Vassilev, N. G.; Tafradjiiska-Hadjiolova, R.; Surcheva, S.; Tchekalarova, J. C. R. Acad. Bulg. Sci. 2016, 69, 513.
(a) Kidwai, M.; Saxena, S.; Khan, M. K. R.; Thukral, S. S. Bioorg. Med. Chem. Lett. 2005, 15, 4295. (b) Kumar, D.; Reddy, V. B.; Sharad, S.; Dube, U.; Kapur, S. Eur. J. Med. Chem. 2009, 44, 3805. (c) Gholipour, S.; Davoodnia, A.; Nakhaei-Moghaddam, M. Chem. Heterocycl. Compd. 2015, 51, 808. [Khim. Geterotsikl. Soedin. 2015, 51, 808.]
Shestopalov, A. M.; Litvinov, Yu. M.; Rodinovskaya, L. A.; Malyshev, O. R.; Semenova, M. N.; Semenov, V. V. ACS Comb. Sci. 2012, 14, 484.
Wang, J.-L.; Liu, D.; Zhang, Z.-J.; Shan, S.; Han, X.; Srinivasula, S. M.; Croce, C. M.; Alnemri, E. S.; Huang, Z. Proc. Natl. Acad. Sci. U. S. A. 2000, 97, 7124.
Gourdeau, H.; Leblond, L.; Hamelin, B.; Desputeau, C.; Dong, K.; Kianicka, I.; Custeau, D.; Boudreau, C.; Geerts, L.; Cai, S.-X.; Drewe, J.; Labrecque, D.; Kasibhatla, S.; Tseng, B. Mol. Cancer Ther. 2004, 3, 1375.
(a) Thareja, S.; Verma, A.; Kalra, A.; Gosain, S.; Rewatkar, P. V.; Kokil, G. R. Acta Pol. Pharm. 2010, 67, 423. (b) Ashok, D.; Lakshmi, B. V.; Ravi, S.; Ganesh, A.; Adam, S. Chem. Heterocycl. Compd. 2015, 51, 462. [Khim. Geterotsikl. Soedin. 2015, 51, 462.]
Mladenović, M.; Mihailović, M.; Bogojević, D.; Matić, S.; Nićiforović, N.; Mihailović, V.; Vuković, N.; Sukdolak, S.; Solujić, S. Int. J. Mol. Sci. 2011, 12, 2822.
Chung, S.-T.; Huang, W.-H.; Huang, C.-K.; Liu, F.-C.; Huang, R.-Y.; Wu, C.-C.; Lee, A.-R. Res. Chem. Intermed. 2016, 42, 1195.
Kamdar, N. R.; Haveliwala, D. D.; Mistry, P. T.; Patel, S. K. Med. Chem. Res. 2011, 20, 854.
(a) Kemnitzer, W.; Jiang, S.; Wang, Y.; Kasibhatla, S.; Crogan-Grundy, C.; Bubenik, M.; Labrecque, D.; Denis, R.; Lamothe, S.; Attardo, G.; Gourdeau, H.; Tseng, B.; Drewe, J.; Cai, S. X. Bioorg. Med. Chem. Lett. 2008, 18, 603. (b) Kemnitzer, W.; Drewe, J.; Jiang, S.; Zhang, H.; Crogan-Grundy, C.; Labreque, D.; Bubenick, M.; Attardo, G.; Denis, R.; Lamothe, S.; Gourdeau, H.; Tseng, B.; Kasibhatla, S.; Cai, S. X. J. Med. Chem. 2008, 51, 417.
Foloppe, N.; Fisher, L. M.; Howes, R.; Potter, A.; Robertson, A. G. S.; Surgenor, A. E. Bioorg. Med. Chem. 2006, 14, 4792.
Kym, P. R.; Kort, M. E.; Coghlan, M. J.; Moore, J. L.; Tang, R.; Ratajczyk, J. D.; Larson, D. P.; Elmore, S. W.; Pratt, J. K.; Stashko, M. A.; Falls, H. D.; Lin, C. W.; Nakane, M.; Miller, L.; Tyree, C. M.; Miner, J. N.; Jacobson, P. B.; Wilcox, D. M.; Nguyen, P.; Lane, B. C. J. Med. Chem. 2003, 46, 1016.
Liu, Y.; Ding, Y. Huaxue Yanjiu Yu Yingyong 1995, 7, 430 [in Chinese].
(a) Sashidhara, K. V.; Palnati, G. R.; Singh, L. R; Upadhyay, A.; Avula, S. R.; Kumar, A.; Kant, R. Green Chem. 2015, 17, 3766. (b) Mulakayala, N.; Rambabu, D.; Raja, M. R.; Chaitanya, M.; Kumar, C. S.; Kalle, A. M.; Krishna, G. R.; Reddy, C. M.; Rao, M. V. B.; Pal, M. Bioorg. Med. Chem. 2012, 20, 759. (c) Goswami, L.; Gogoi, S.; Gogoi, J.; Boruah, R. K.; Boruah, R. C.; Gogoi, P. ACS Comb. Sci. 2016, 18, 253.
Zhi, L.; Tegley, C. M.; Pio, B.; Edwards, J. P.; Motamedi, M.; Jones, T. D.; Marschke, K. B.; Mais, D. E.; Risek, B.; Schrader, W. T. J. Med. Chem. 2003, 46, 4104.
(a) Elmore, S. W.; Pratt, J. K.; Coghlan, M. J.; Mao, Y.; Green, B. E.; Anderson, D. D.; Stashko, M. A.; Lin, C. W.; Falls, D.; Nakane, M.; Miller, L.; Tyree, C. M.; Miner, J. N.; Lane, B. Bioorg. Med. Chem. Lett. 2004, 14, 1721. (b) Ku, Y. Y.; Grieme, T.; Raje, P.; Sharma, P.; Morton, H. E.; Rozema, M.; King, S. A. J. Org. Chem. 2003, 68, 3238.
(a) Stadlbauer, W. Monatsh. Chem. 1987, 118, 1297. (b) Colin, G. M.; Gear, J. R. J. Nat. Prod. 1993, 56, 1402.
Paul, S.; Das, A. R. Tetrahedron Lett. 2012, 53, 2206.
Ahmed, N.; Babu, B. V.; Singh, S.; Mitrasinovic, P. M. Heterocycles 2012, 85, 1629.
Nandi, S.; Gupta, A.; Pal, A. K. Lett. Org. Chem. 2017, 14, 291.
Yahya-Meymandi, A.; Nikookar, H.; Moghimi, S.; Mahdavi, M.; Firoozpour, L.; Asadipour, A.; Ranjbar, R. P.; Foroumadi, A. J. Iran. Chem. Soc. 2017, 14, 771.
Curran, D. P.; Liu, H. J. Am. Chem. Soc. 1992, 114, 5863.
Williams, D. R.; Lowder, P. D.; Gu, Y.-G. Tetrahedron Lett. 1997, 38, 327.
Brickner, S. J. Chem. Ind. 1997, 131.
Kozikowski, A. P.; Campiani, G.; Sun, L.-Q.; Wang, S.; Saxena, A.; Doctor, B. P. J. Am. Chem. Soc. 1996, 118, 11357.
Cox, R. J.; O'Hagan, D. J. Chem. Soc., Perkin Trans. 1 1991, 2537.
Sayahi, M. H.; Saghanezhad, S. J.; Mahdavi, M. Res. Chem. Intermed. 2018, 44, 739.
Firoozpour, L.; Nikookar, H.; Moghimi, S.; Mahdavi, M.; Asadipour, A.; Ranjbar, P. R; Foroumadi, A. Heterocycl. Commun. 2017, 23, 305.
(a) Olyaei, A.; Sadeghpour, M.; Zarnegar, M. Chem. Heterocycl. Compd. 2013, 49, 1374. [Khim. Geterotsikl. Soedin. 2013, 1474.] (b) Olyaei, A.; Rahbarian, F.; Sadeghpour, M. Chem. Heterocycl. Compd. 2015, 51, 899. [Khim. Geterotsikl. Soedin. 2015, 51, 899.] (c) Olyaei, A.; Shafie, Z.; Sadeghpour, M. Tetrahedron Lett. 2018, 59, 3567. b Olyaei, A.; Saraei, M.; Khoeiniha, R. Synlett 2018, 1589. c Olyaei, A.; Shahsavari, M. S.; Sadeghpour, M. Res. Chem. Intermed. 2018, 44, 943. d Khoeiniha, R.; Olyaei, A.; Saraei, M. Synth. Commun. 2018, 48, 155. e Khoeiniha, R.; Olyaei, A.; Saraei, M. J. Heterocycl. Chem. 2017, 54, 1746. f Olyaei, A.; Javarsineh, S.; Sadeghpour, M. Chem. Heterocycl. Compd. 2018, 54, 934. [Khim. Geterotsikl. Soedin. 2018, 54, 934.]
The authors thank Research Council of the Payame Noor University for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2019, 55(11), 1104–1110
Electronic supplementary material
ESM 1
(PDF 3874 kb)
Rights and permissions
About this article
Cite this article
Olyaei, A., Ebrahimi, R.M., Adl, A. et al. Green synthetic approach toward new chromeno[4,3-b]quinoline and chromeno[4,3-b]pyridine derivatives. Chem Heterocycl Comp 55, 1104–1110 (2019). https://doi.org/10.1007/s10593-019-02585-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-019-02585-4