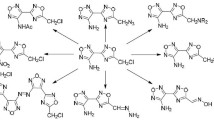
5-Aryl-2-furaldehydes, obtained by furfural arylation with arenediazonium salts, react with ethyl acetoacetate or acetylacetone and (thio)-urea in the presence of FeCl3·6H2O as a catalyst. A series of ethyl 4-(5-aryl-2-furyl)-6-methyl-2-oxo(thioxo)-1,2,3,4-tetrahydropyrimidine-5-carboxylates was obtained.


Similar content being viewed by others
References
a) Multicomponent Reactions in Organic Synthesis; Zhu, J.; Wang, Q.; Wang, M., Eds.; Wiley: Weinheim, 2015. a Müller, T. J. J. Beilstein J. Org. Chem. 2014, 10, 115. b Synthesis of Heterocycles via Multicomponent Reactions II; Orru, R. V. A.; Ruijter, E., Eds.; Springer, 2010. c Banfi, L.; Basso, A.; Lambruschini, C.; Moni, L.; Riva, R. Chem. Heterocycl. Compd. 2017, 53, 382. [Khim. Geterotsikl. Soedin. 2017, 53, 382.] (e) Orru, R. V. A.; de Greef, M. Synthesis 2003, 1471. d Zhu, J. Eur. J. Org. Chem. 2003, 7, 1133. e Burke, M. D.; Schreiber, S. L. Angew. Chem., Int. Ed. 2004, 43, 46. f Ramon, D. J.; Yus M. Angew. Chem., Int. Ed. 2005, 44, 1602. g Golantsov, N. E.; Nguyen, H. M.; Varlamov, A. V.; Aksenov, A. V.; Voskressensky, L. G. Chem. Heterocycl. Compd. 2017, 53, 446. [Khim. Geterotsikl. Soedin. 2017, 53, 446.] (j) Dömling, A. Chem. Rev. 2006, 106, 17. h Komykhov, S. A.; Bondarenko, A. A.; Musatov, V. I.; Diachkov, M. V.; Gorobets, N. Yu.; Desenko, S. M. Chem. Heterocycl. Compd. 2017, 53, 378. [Khim. Geterotsikl. Soedin. 2017, 53, 378.] (l) Nielsen, T. E.; Schreiber, S. L. Angew. Chem., Int. Ed. 2008, 47, 48. i Dotsenko, V. V.; Ismiev, A. I.; Khrustaleva, A. N.; Frolov, K. A.; Krivokolysko, S. G.; Chigorina, E. A.; Snizhko, A. P.; Gromenko, V. M.; Bushmarinov, I. S.; Askerov, R. K.; Pekhtereva, T. M.; Suykov, S. Yu.; Papayanina, E. S.; Mazepa, A. V.; Magerramov, A. M. Chem. Heterocycl. Compd. 2016, 52, 473. [Khim. Geterotsikl. Soedin. 2016, 52, 473.]
(a) Weber, L. Drug Discovery Today 2002, 7, 143. (b) Dömling, A. Curr. Opin. Chem. Biol. 2002, 6, 306. (c) Gore, R. P.; Rajput, A. P. Drug Invent. Today 2013, 5, 148. (d) Spandl, R. J.; Bender, A.; Spring, D. R. Org. Biomol. Chem. 2008, 6, 1149. (e) Tan, D. S. Nat. Chem. Biol. 2005, 1, 74. (f) Dömling, A. Curr. Opin. Chem. Biol. 2000, 4, 318. (g) Glasnov, T. N.; Vugts, D. J.; Koningstein, M. M.; Desai, B.; Fabian, W. M. F.; Orru, R. V. A.; Kappe, C. O. QSAR Comb. Sci. 2006 , 25, 509. (h) Dolle, R. E.; Nelson, K. H. J. Comb. Chem. 1999, 1, 235.
(a) Lee, D.; Sello, J. K.; Schreiber, S. L. Org. Lett. 2000, 2, 709. (b) Paravidino, M.; Bon, R. S.; Scheffelaar, R.; Vugts, D. J.; Znabet, A.; Schmitz, R. F.; de Kanter, F. J. J.; Lutz, M.; Spek, A. L.; Groen, M. B.; Orru, R. V. A. Org. Lett. 2006, 8, 5369. (c) Groenendaal, B.; Ruijter, E.; de Kanter, F. J. J.; Lutz, M.; Spek, A. L.; Orru, R. V. A. Org. Biomol. Chem. 2008, 6, 3158. (d) Khanetskyy, B.; Dallinger, D.; Kappe C. O. J. Comb. Chem. 2004, 6, 884.
(a) Kappe, C. O. Acc. Chem. Res. 2000, 33, 879. (b) Kappe, C. O. J. Org. Chem. 1997, 6, 7201. (c) Kappe, C. O.; Stadler, A. Org. React. 2004, 63, 1. (d) Vdovina S. V.; Mamedov V. A. Russ. Chem. Rev. 2008, 77, 1017. [Usp. Khim. 2008, 77, 1091.] (e) Terentjeva, S.; Muceniece, D.; Lusis, V. Chem. Heterocycl. Compd. 2014, 49, 1757. [Khim. Geterotsikl. Soedin. 2013, 1896.] (f) Chebanov, V. A.; Desenko, S. M. Chem. Heterocycl. Compd. 2012, 48, 566. [Khim. Geterotsikl. Soedin. 2013, 607.] (g) Kolosov, M. A.; Al-Ogaili, M. J. K.; Parkhomenko, V. S.; Orlov, V. D. Chem. Heterocycl. Compd. 2014, 49, 1484. [Khim. Geterotsikl. Soedin. 2013, 1599.] (h) Kurmach, M. N.; Ryabitskiy, A. B.; Britsun, V. N. Chem. Heterocycl. Compd. 2014, 49, 1770. [Khim. Geterotsikl. Soedin. 2013, 1910.] (i) Jauk, B.; Belaj, F.; Kappe C. O. J. Chem. Soc., Perkin Trans. 1 1999, 307. e Maiboroda, O.; Simurova, N. Chem. Chem. Technol. 2016, 10, 279. f Simurova, N.; Maiboroda, O. Chem. Heterocycl. Compd. 2017, 53, 413. [Khim. Geterotsikl. Soedin. 2017, 53, 413.] (l) Gümüş, M. K.; Gorobets, N. Yu.; Sedash, Yu. V.; Chebanov, V. A.; Desenko, S. M. Chem. Heterocycl. Compd. 2017, 53, 1261. [Khim. Geterotsikl. Soedin. 2017, 53, 1261.]
(a) Lagoja, I. M. Chem. Biodiversity 2005, 2, 1. (b) Brown, D. J.; Evans, R. F.; Cowden, W. B.; Fenn, M. D. In Chemistry of Heterocyclic Compounds; Wiley: New York, 1994, vol. 52, p. 47. (c) Kappe, C. O.; Fabian, W. M. F.; Semones, M. A. Tetrahedron 1997, 53, 2803. (d) Patil, A. D.; Kumar, N. V.; Kokke, W. C.; Bean, M. F.; Freyer, A. J.; De Brosse, C.; Mai, S.; Truneh, A.; Faulkner, D. J.; Carte, B.; Breen, A. L.; Hertzberg, R. P.; Johnson, R. K.; Westley, J. W.; Potts, B. C. M. J. Org. Chem. 1995, 60, 1182. (e) Crespo, A.; El Maatougui, A.; Azuaje, J.; Escalante, L.; Majellaro, M.; Loza, M. I.; Brea, J.; Cadavid, M. I.; Gutiérrez-de-Terán, H.; Sotelo, E. Chem. Heterocycl. Compd. 2017, 53, 316. [Khim. Geterotsikl. Soedin. 2017, 53, 316.]
(a) Kappe, C. O. Eur. J. Med. Chem. 2000, 35, 1043. (b) Blackburn, C.; Guan, B.; Brown, J.; Cullis, C.; Condon, S. M.; Jenkins, T. J.; Peluso, S.; Ye, Y.; Gimeno, R. E.; Punreddy, S.; Sun, Y.; Wu, H.; Hubbard, B.; Kaushik, V.; Tummino, P.; Sanchetti, P.; Sun, D. Y.; Daniels, T.; Tozzo, E.; Balani, S. K.; Raman, P. Bioorg. Med. Chem. Lett. 2006, 16, 3504. (c) Hurst, E. W.; Hull, R. J. Med. Pharm. Chem. 1961, 3, 215. (d) Mayer, T. U.; Kapoor, T. M.; Haggarty, S. J.; King, R. W.; Schreiber, S. I.; Mitchison, T. J. Science 1999, 286, 971. (e) Atwal, K. S.; Swanson, B. N.; Unger, S. E.; Floyd, D. M.; Moreland, S.; Hedberg, A.; O'Reilly, B. C. J. Med. Chem. 1991, 34, 806. (f) Rovnyak, G. C.; Atwal, K. S.; Hedberg, A.; Kimball, S. D.; Moreland, S.; Gougoutas, J. Z.; O'Reilly, B. C.; Schwartz, J.; Malley, M. F. J. Med. Chem. 1992, 35, 3254. (g) Suresh; Sandhu, J. S. ARKIVOC, 2012, (i), 66.
(a) Organic-Chemical Drugs and Their Synonyms; Negwer; M.; Scharnow, H.-G., Eds.; Wiley, 2001, p. 3406 (b) The Dictionary of Drugs: Chemical Data, Structures and Bibliographies; Elks, J.; Ganellin, C. R., Eds.; Springer, 1990. pp. 294, 346. b Wang, G.; Wang, X.; Yu, H.; Wei, S.; Williams, N.; Holmes, D. L.; Halfmann, R.; Naidoo, J.; Wang, L.; Li, L.; Chen, S.; Harran, P.; Lei, X.; Wang, X. Nat. Chem. Biol. 2013, 9, 84. c Jiang, S.; Tala, S. R.; Lu, H.; Abo-Dya, N. E.; Avan, I.; Gyanda, K.; Lu, L.; Katritzky, A. R.; Debnath, A. K. J. Med. Chem. 2011, 54, 572. d Hansen, S. W.; Erichsen, M. N.; Fu, B.; Bjørn-Yoshimoto, W. E.; Abrahamsen, B.; Hansen, J. C.; Jensen, A. A.; Bunch L. J. Med. Chem. 2016, 59, 8757. e Horak, Yu. I.; Matiychuk, V. S.; Obushak, M. D.; Kutsyk, R. V.; Lytvyn, R. Z.; Kurovets, L. M. Ukr. Bioorg. Acta 2008, 6, 49.
(a) Lauro, G.; Strocchia, M.; Terracciano, S.; Bruno, I.; Fischer, K.; Pergola, C.; Werz, O.; Riccio, R.; Bifulco, G. Eur. J. Med. Chem. 2014, 80, 407. (b) Strocchia, M.; Terracciano, S.; Chini, M. G.; Vassallo, A.; Vaccaro, M. C.; Dal Piaz, F.; Leone, A.; Riccio, R.; Bruno, I.; Bifulco, G. Chem. Commun. 2015, 51, 3850. (c) Terracciano, S.; Lauro, G.; Strocchia, M.; Fischer, K.; Werz, O.; Riccio, R.; Bruno, I.; Bifulco, G. ACS Med. Chem. Lett. 2015, 6, 187.
(a) Obushak, N. D.; Lesyuk, A. I.; Ganushchak, N. I.; Mel'nik, G. M.; Zavalii, P. Yu. J. Org. Chem. USSR 1986, 22, 2093. [Zh. Org. Khim. 1986, 22, 2331.] (b) Obushak, N. D.; Ganushchak, N. I.; Lesyuk, A. I.; Dzikovskaya, L. M.; Kisilitsa, P. P. J. Org. Chem. USSR 1990, 26, 748. [Zh. Org. Khim. 1990, 26, 873.] (c) Obushak, N. D.; Gorak, Yu. I.; Matiichuk, V. S.; Lytvyn, R. Z. Russ. J. Org. Chem. 2008, 44, 1689. [Zh. Org. Khim. 2008, 44, 1712.] (d) Gorak, Yu. I.; Obushak, N. D.; Matiichuk, V. S.; Lytvyn, R. Z. Russ. J. Org. Chem. 2009, 45, 541. [Zh. Org. Khim. 2009, 45, 555.] (e) Obushak, N. D.; Lesyuk, A. I.; Gorak, Yu. I.; Matiichuk, V. S. Russ. J. Org. Chem. 2009, 45, 1375. [Zh. Org. Khim. 2009, 45, 1388.]
(a) Belsky, V. K.; Stash, A. I.; Koval'chukova, O. V.; Strashnova, S. B.; Shebaldina, L. S.; Zaitsev, B. E.; Pleshakov, V. G. Crystallogr. Rep. 2003, 48, 606. [Kristallografiya 2003, 48, 657.] (b) Wang, H.-Y. Acta Crystallogr., Sect. E: Struct. Rep. Online 2010, E66, o2822. b Vega-Teijido, M.; Zukerman-Schpector, J.; Nunes, F. M.; Gatti, P. M.; Stefani, H. A.; Caracelli, I. Z. Kristallogr.–Cryst. Mater. 2007, 222, 705. c Novina, J. J.; Vasuki, G.; Suresh, M.; Viswanathan, V.; Velmurugan, D. Int. Union Crystallogr. Data 2016, 1, x161937.
(a) McClure, M. S.; Roschangar, F.; Hodson, S. J.; Millar, A.; Osterhout, M. H. Synthesis 2001, 1681. (b) Hosoya, T.; Aoyama, H.; Ikemoto, T.; Kihara, Y.; Hiramatsu, T.; Endo, M.; Suzuki, M. Bioorg. Med. Chem. 2003, 11, 663.
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr. 2015, A71, 3.
Sheldrick, G. M. Acta Crystallogr., Sect. C: Struct. Chem. 2015, C71, 3.
This publication was supported by the Ministry of Education and Science of Ukraine (project 0118U003610) and by the Ministry of Education and Science of the Russian Federation (project 4.1154.2017/4.6).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii,2018, 54(5), 545–549
Rights and permissions
About this article
Cite this article
Vakhula, A.R., Horak, Y.I., Lytvyn, R.Z. et al. 5-Aryl-2-furaldehydes in the synthesis of tetrahydropyrimidinones by Biginelli reaction. Chem Heterocycl Comp 54, 545–549 (2018). https://doi.org/10.1007/s10593-018-2301-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-018-2301-3