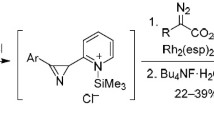
Rh2(OAc)4-catalyzed Wolff rearrangement of methyl 2-diazo-3-(4-methoxyphenyl)-3-oxopropanoate gave methoxycarbonyl(4-methoxyphenyl) ketene that was added to 2Н-azirines, resulting in opening of the three-membered ring at the N–C(2) bond and leading to the formation of 3,4-dihydro-2Н-pyrrol-2-one derivatives. Depending on the structure of the obtained products, they isomerized to the more stable 1Н-pyrrol-2(3H)-one derivatives or added a water molecule at the C=N bond.

Similar content being viewed by others
References
Kirmse, W. Eur. J. Org. Chem. 2002, 2193.
Allen, A. D.; Tidwell, T. T. Chem. Rev. 2013, 113, 7287.
Khlebnikov, A. F.; Novikov, M. S. In Topics in Heterocyclic Chemistry: Synthesis of 4- to 7-Membered Heterocycles by Ring Expansion; D'hooghe, M.; Ha, H.-J., Eds.; Springer: Geneva, 2016, Vol. 41, p. 143.
Khlebnikov, A. F.; Novikov, M. S. Tetrahedron 2013, 69, 3363.
Kascheres, A.; Nunes, J., Jr.; Brandão, F. Tetrahedron 1997, 53, 7089.
Khlebnikov, A. F.; Novikov, M. S.; Pakalnis, V. V.; Yufit, D. S. J. Org. Chem. 2011, 76, 9344.
Khlebnikov, A. F.; Novikov, M. S.; Pakalnis, V. V.; Iakovenko, R. O.; Yufit, D. S. Beilstein J. Org. Chem. 2014, 10, 784.
Pandit, R. P.; Lee, Y. R. Org. Biomol. Chem. 2014, 12, 4407.
Zhang, Z.; Tang, M.; Zang, L.; Zou, L.-H.; Li, J. Tetrahedron Lett. 2016, 57, 5681.
Lindsay, V. N. G.; Nicolas, C.; Charette, A. B. J. Am. Chem. Soc. 2011, 133, 8972.
Marcoux, D.; Goudreau, S. R.; Charette, A. B. J. Org. Chem. 2009, 74, 8939.
Sandridge, M. J.; France, S. Org. Lett. 2016, 18, 4218.
McLarney, B. D.; Cavitt, M. A.; Donnell, T. M.; Musaev, D. G.; France, S. Chem.–Eur. J. 2017, 23, 1129.
Lévesque, É.; Campeau, L.-C.; Gauvreau, D. Synlett 2010, 3086.
Kim, J.-A.; Seo, Y. J.; Kang, S.; Han, J.; Lee, H.-K. Chem. Commun. 2014, 50, 13706.
Jiang, Y.; Khong, V. Z. Y.; Lourdusamy, E.; Park, C.-M. Chem. Commun. 2012, 48, 3133.
Fowler, F. W.; Hassner, A.; Levy, L. A. J. Am. Chem. Soc. 1967, 89, 2077.
Wang, X.; Zhang, C.-Y.; Tu, H.-Y.; Zhang, A.-D. Eur. J. Org. Chem. 2016, 5243.
Rostovskii, N. V.; Novikov, M. S.; Khlebnikov, A. F.; Khlebnikov, V. A.; Korneev, S. M. Tetrahedron 2013, 69, 4292.
Cox, G. G.; Miller, D. J.; Moody, C. J.; Sie, E.-R. H. B.; Kulagowski, J. J. Tetrahedron 1994, 50, 3195.
Tidwell, T. T. Ketenes; Wiley Interscience: Hoboken, 2006, 2nd ed., 648 p.
Yu, J.; Tian, J.; Zhang, C. Adv. Synth. Catal. 2010, 352, 531.
Schulthess, A. H.; Hansen, H.-J. Helv. Chim. Acta 1981, 64, 1322.
Pawlowski, M.; Wojtasiewicz, K.; Maurin, J. K.; Leniewski, A.; Blachut, D.; Czarnocki, Z. Heterocycles 2007, 71, 1743.
Fedoseev, S. V.; Ershov, O. V.; Belikov, M. Yu.; Lipin, K. V.; Bardasov, I. N.; Nasakin, O. E.; Tafeenko, V. A. Tetrahedron Lett. 2013, 54, 2143.
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. J. Appl. Crystallogr. 2009, 42, 339.
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, A64, 112.
This work was performed with financial support from the Russian Science Foundation (project No. 17-13-01078). The analysis of obtained compounds was performed at the Saint Petersburg State University resource centers “Magnetic Resonance Research Centre” and “Chemical Analysis and Materials Research Centre”.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2017, 53(9), 985–988
Electronic supplementary material
ESM 1
(PDF 3675 kb)
Rights and permissions
About this article
Cite this article
Rostovskii, N.V., Novikov, M.S., Khlebnikov, A.F. et al. Two-atom azirine ring expansion reaction of methyl 2-diazo-3-(4-methoxyphenyl)-3-oxopropanoate via a dirhodium tetraacetate-catalyzed Wolff rearrangement. Chem Heterocycl Comp 53, 985–988 (2017). https://doi.org/10.1007/s10593-017-2160-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-017-2160-3