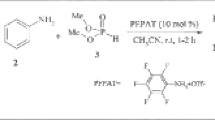
Air-stable and crystalline tetraaminophosphonium tetrafluoroborates possessing chiral, enantiomerically pure 1,2-diamine moiety have been synthesized by a three-step sequential one-pot approach. The tetrafluoroborate salts can be purified by recrystallization or chromatography and subsequently converted to the phosphazene bases by treatment with t-BuOK. Basicity values in tetrahydrofuran have been measured for the obtained phosphazene bases by means of spectrophotometric titration.

Similar content being viewed by others
References
(a) Herzberger, J.; Niederer, K.; Pohlit, H.; Seiwert, J.; Worm, M.; Wurm, F. R.; Frey, H. Chem. Rev. 2016, 116, 2170. (b) Kondo, Y. In Superbases for Organic Synthesis; Shikawa, T., Ed.; Wiley: Chichester, 2009, ch. 5. (c) Boileau, S.; Illy, N. Prog. Polym. Sci. 2011, 36, 1132. (d) Solladié-Cavallo, A.; Roje, M.; Welter, R.; Šunjić, V. J. Org. Chem. 2004, 69, 1409.
(a) Lee, Y.-J.; Lee, J.; Kim, M.-J.; Jeong, B.-S.; Lee, J.-H.; Kim, T.-S.; Lee, J.; Ku, J.-M.; Jew, S.-S.; Park, H.-G. Org. Lett. 2005, 7, 3207. (b) Solladié-Cavallo, A.; Crescenzi, B. Synlett 2000, 327.
(a) Brunel, J. M.; Legrand, O.; Reymond, S.; Buono, G. J. Am. Chem. Soc. 1999, 121, 5807. (b) Takeda, T.; Terada, M. J. Am. Chem. Soc. 2013, 135, 15306. (c) Uraguchi, D.; Ito, T.; Ooi, T. J. Am. Chem. Soc. 2009, 131, 3836. (d) Uraguchi, D.; Yoshioka, K.; Ueki, Y.; Ooi, T. J. Am. Chem. Soc. 2012, 134, 19370. (e) Uraguchi, D.; Tsutsumi, R.; Ooi, T. J. Am. Chem. Soc. 2013, 135, 8161.
(a) Schwesinger, R.; Schlemper, H.; Hasenfratz, C.; Willaredt, J.; Dambacher, T.; Breuer, T.; Ottaway, C.; Fletschinger, M.; Boele, J.; Fritz, H.; Putzas, D.; Rotter, H. W.; Bordwell, F. G.; Satish, A. V.; Ji, G.-Z.; Peters, E.-M.; Peters, K.; von Schnering, H. G.; Walz, L. Liebigs Ann. 1996, 1055. (b) Köhn, U.; Schulz, M.; Schramm, A.; Günther, W.; Görls, H.; Schenk, S.; Anders, E. Eur. J. Org. Chem. 2006, 4128. (c) Alexandrova, A. V.; Masek, T.; Polyakova, S. M.; Cisarova, I.; Saame, J.; Leito, I.; Lyapkalo, I. M. Eur. J. Org. Chem. 2013, 9, 1811.
(a) Alajarín, M.; López-Leonardo, C.; Berná, J. Tetrahedron 2006, 62, 6190. (b) Terada, M.; Goto, K.; Oishi, M.; Takeda, T.; Kwon, E.; Kondoh, A. Synlett 2013, 24, 2531. (c) Kögel, J. F.; Kneusels, N.-J.; Sundermeyer, J. Chem. Commun. 2014, 50, 4319.
(a) Vedejs, E.; Donde, Y. J. Org. Chem. 2000, 65, 2337. (b) Nettekoven, U.; Widhalm, M.; Kamer, P. C. J.; van Leeuwen, P. W. N. M.; Mereiter, K.; Lutz, M.; Spek, A. L. Organometallics 2000, 19, 2299. (c) Colby, E. A.; Jamison,T. F. J. Org. Chem. 2003, 68, 156.
Tolman, C. A. Chem. Rev. 1977, 77, 313.
(a) Kaljurand, I.; Rodima, T.; Leito, I.; Koppel, I. A.; Schwesinger, R. J. Org. Chem. 2000, 65, 6202. (b) Rodima, T.; Kaljurand, I.; Pihl, A.; Mäemets, V.; Leito, I.; Koppel, I. A. J. Org. Chem. 2002, 67, 1873. (c) Kaljurand, I.; Kütt, A.; Sooväli, L.; Rodima, T.; Mäemets, V.; Leito, I.; Koppel, I. A. J. Org. Chem. 2005, 70, 1019. (d) Kaljurand, I.; Rodima, T.; Pihl, A.; Mäemets, V.; Leito, I.; Koppel, I. A.; Mishima, M. J. Org. Chem. 2003, 68, 9988. (e) Kaljurand, I.; Saame, J.; Rodima, T.; Koppel, I.; Koppel, I. A.; Kögel, J. F.; Sundermeyer, J.; Köhn, U.; Coles, M. P.; Leito, I. J. Phys. Chem. A 2016, 120, 2591.
Alexakis, A.; Mutti, S.; Mangeney, P. J. Org. Chem. 1992, 57, 1224.
Alexakis, A.; Aujard, I.; Kanger, T.; Mangeney, P. Org. Synth. 2004, 10, 312; 1999, 76, 23.
Mark, V. Org. Synth. 1966, 46, 42; 1973, 5, 602.
Bottaro, J. C.; Penwell, P. E.; Schmitt, R. J. Synth. Commun. 1997, 27, 1465.
Andersen, J.; Madsen, U.; Björkling, F.; Liang, X. Synlett 2005, 2209.
G. M. Sheldrick, SHELXL-97: Program for the Solution of Crystal Structures. University of Gőttingen (1997).
Authors thank Dr. S. Belyakov (Latvian Institute of Organic Synthesis) for X-ray crystallographic analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
The Supplementary information file containing results of X-ray crystallographic analysis is available at http://link.springer.com/journal/10593.
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2016, 52(8), 541–545
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 325 kb)
Rights and permissions
About this article
Cite this article
Priede, M., Priede, E., Saame, J. et al. Synthesis of Chiral Phosphazene Bases. Chem Heterocycl Comp 52, 541–545 (2016). https://doi.org/10.1007/s10593-016-1927-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-016-1927-2