Abstract
Pond physical characteristics (connectivity, hydroperiod) have shown to be highly relevant in explaining species presence, reproductive success, and survival in breeding-pond amphibians. However, few studies have addressed the influence that these factors may have on the genetic variability of pond populations. We examined genetic variation at 11 microsatellite loci in Iberian endemic, the pygmy newt (Triturus pygmaeus), from 58 breeding ponds in the Doñana National Park (Andalusia), including both temporary ponds and artificially deepened ponds that remain wet during the whole year. Temporary ponds are located in the North part of the region where the surrounding habitat-wet meadows-facilitates the connectivity among populations, whereas the deepest ponds (‘zacallones’) are located in the southern edge embedded in a matrix of unsuitable habitat (thickets and dry underbrush). We investigated genetic diversity and structure within and among ponds. Our results show that both regions (Doñana-North and Doñana-South) are well-differentiated and form two main clusters. We found higher genetic diversity within ponds from the North region, which also exhibited a higher degree of genetic admixture in comparison with populations from the southern edge. Although we found an isolation-by-distance pattern within each cluster, it arose due to the effect of a few isolated ponds located on the edge of each zone, suggesting the existence of substantial gene flow between ponds in the core area. According to our findings, landscape’s permeability to movement (pond connectivity) may constitute a more important factor than hydroperiod length in determining the genetic diversity and viability of pygmy newt populations in this area. Although Doñana populations show a good state, more peripheral and isolated populations present a more worrisome condition as a result of fragmentation and thus, require conservation efforts. Our study provides key insights that could help guide management practices of this threatened and poorly-studied salamander.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pygmy newt (or southern marbled newt) Triturus pygmaeus is a species of salamander in the family Salamandridae (subfamily Pleurodelinae) whose geographical range is restricted to the southwest of the Iberian Peninsula (García-París 2002). Thus, the pygmy newt is one of the European vertebrates with a southernmost distribution together with the Portuguese smooth newt (Lissotriton maltzani) and the Betic midwife toad (Alytes dickhilleni) (Arntzen and García-París 1995; Sequeira et al. 2020). The pygmy newt was formerly considered a subspecies of the marbled newt Triturus marmoratus, which inhabits the northern part of the Iberian Peninsula as well as central and southern France (García-París et al. 2001). Hence, Triturus marmoratus and T. pygmaeus are essentially parapatric, a common feature in other Triturus species whose ranges only slightly overlap (Espregueira Themudo 2010). The contact zone marmoratus-pygmaeus runs along an east–west belt from Madrid (Spain) to Abrantes (Portugal). In Portugal, the location of one of the contact zones in which hybrids have been detected coincides with the Tagus river, which seems to act as a barrier to dispersal and delimits the boundaries of both species (Espregueira Themudo and Arnyzen 2007; Arntzen et al. 2021).
Triturus pygmaeus inhabits temperate forests, Mediterranean-type shrubby vegetation, rivers, intermittent freshwater marshes, pastureland, and permanent and temporary ponds. It is catalogued as ‘Near Threatened’ (IUCN 2021) due to a population decline (~ 30%) in the last decades linked to habitat loss as a consequence of global warming and land-use changes (e.g., expansion of irrigated agriculture and its knock-on effects) (Reques et al. 2006). Temporary ponds and other aquatic environments with recurrent periods of desiccation are optimal habitats (and/or breeding sites) for newts and many other vertebrate and macroinvertebrate species (Collinson et al. 1995; Díaz-Paniagua 1990; Nicolet et al. 2004; Díaz-Paniagua et al. 2005; Florencio et al. 2009). Mediterranean temporary ponds hold a different biota than permanent waters do, and thus they are exceptionally singular in terms of species and community structure (Boix et al. 2008; Díaz-Paniagua et al. 2010). Despite this, these microhabitats have traditionally received less attention than large water bodies (i.e., extensive wetlands) by conservationists and ecologists. Nevertheless, during the last decades, awareness of the conservation value of these fragile ecosystems has been made to the point of being considered priority habitats by the European Union (code 3170, ‘Habitats directive’ 92/43/EEC) (Bagella et al. 2016).
The pygmy newt is one of the Iberian endemics that can be found among the more than 3,000 water bodies that conform to the system of temporary ponds of the Doñana region, in southern Spain. Doñana is considered the most relevant wetland area in Spain, which extends along the coastal plain of the Gulf of Cádiz from the left bank of the estuary of the Guadalquivir river to the estuary of the Tinto river. The Doñana region comprises several territories with a different degree of protection covering an extension of over 100,000 ha; a Biological Reserve since 1964, a National Park (the ‘core area’, designated as a Ramsar site in 1982 and a World Heritage Site by UNESCO in 1995) and a Natural Park created as a surrounding protective (‘buffer’) area in 1989 (Serrano et al. 2006). Due to its proximity to culturally and economically critical locations, Doñana has been shaped by people for centuries (Green et al. 2018). Hence, despite its incalculable conservation value, this region has been constantly under threat from the drainage of marshlands, use of groundwater for intensive agriculture (rice and strawberry fields) and water pollution (Fernández-Delgado 2017). The coexistence of natural ecosystems and human-modified environments also characterize the aquatic systems of Doñana, in which converge natural water bodies (i.e., temporary ponds locally known as lagoons) and water holes artificially deepened (known as ‘zacallones’) to guarantee the availability of water for cattle throughout the year, especially in the summer. Consequently, the system of ponds in Doñana largely varies in conditions like connectivity and hydroperiod (i.e., length of the time a pond holds water). For instance, some ponds have a short hydroperiod (they are wet only for 4–6 months), whereas many others -mainly the so-called ‘zacallones’-have water even in August due to its greater depth.
Pond physical characteristics have shown to be highly relevant in explaining species presence, reproductive success, and population persistence in pond-breeding amphibians (e.g., pond size: Wang et al. 2011; Semlitsch et al. 2015; Torres et al. 2015; pond isolation: Ribeiro et al. 2011; pond hydroperiod: Semlitsch et al. 1996). In turn, genetic variability is likely to be affected by these factors as well since small and isolated populations usually exhibit little genetic variation and high levels of inbreeding (Keller and Waller 2022). Inbreeding depression might be more pronounced in stressful environments (i.e., those subjected to perturbation regimes) compared to benign conditions as some previous studies have shown (e.g., Lesbarrères et al. 2005; Fox and Reed 2011). Specifically, the environmental stress acts on natural populations in different ways: (i) randomly, strongly reducing the size of the populations and causing, as a consequence, the loss of a random number of alleles, leading to a reduction of genetic variability and heterozygosity (‘genetic erosion’); and (ii) selectively, causing different mortality rates for the various genotypes, lowering the abundance of the less resistant ones and, therefore, changing the genotypic frequencies in the population in response to selection (Cimmaruta et al. 2003). Although it is well established that changes in hydroperiod can be an important environmental stressor for amphibians during their development and affect several fitness traits including survival (Tejedo et al. 2010; Branelly et al. 2019) and dispersal propensity (Tournier et al. 2017; Cayuela et al. 2020a), few studies have evaluated the effect of interannual ephemerality on the genetic variability of populations, which may underlie the observed relationships between fitness and environmental harshness (but see Watts et al. 2015; Cayuela et al. 2020a). In addition, pond drying may have far-reaching consequences on the evolutionary forces involved in the migration-selection-drift balance.
In the present study, we analyzed the genetic diversity and differentiation of 58 breeding pond populations of T. pygmaeus at the Doñana natural reserve, using eleven polymorphic microsatellite loci. These ponds differ in hydroperiod duration and connectivity; whereas those located in the North part remain flooded for 3–6 months (lagoons) and are surrounded by meadows tolerant of recurrent flood events, those located at the South extreme of the reserve (‘zacallones’) hold water for the whole year and are embedded within a matrix of dry vegetation-pine woodlands- (Gómez-Rodríguez et al. 2011; Díaz-Paniagua et al. 2016) (see Study area section). Thus, one would expect that individuals from northern populations to be more time-constrained and be forced to exhibit faster development times but, in turn, would have more facilities to disperse successfully, which can reduce genetic differentiation among populations (FST) and ultimately increase genetic diversity. Whereas southern populations would exhibit higher within-population survival rates (since ‘zacallones’ remain wet) but lower connectivity (i.e., reduced gene flow) among them, which can exacerbate inbreeding depression (higher FIS values). Thus, in both areas, newts must face challenging conditions (stressful conditions during development, dispersal constraints post-development) which might impair their effective population size as a result of either increased mortality or lack of immigration, respectively. In a further step, we compared the genetic diversity of these populations with that of other isolated populations located at northern latitudes. In this way, we determined if newts from Doñana, which are conspicuously smaller in comparison with individuals from nearby localities with similar climatic characteristics (Díaz-Paniagua et al. 1996), are also distinctive in terms of genetic variability. Since these peripheral populations do not conform to an extensive pond network, we would expect they exhibit lower genetic diversity in comparison with that of the Doñana populations.
Materials and methods
Study area
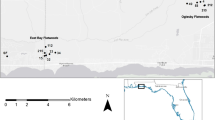
The Doñana region (Huelva, Andalusia, SW Spain) covers a groundwater discharge surface of about 350 km2 in which four different ecosystems predominate; fixed and mobile dunes, beaches, Mediterranean scrub woodland and maquis, and marshlands. The Doñana marshes are flooded by autumn and winter rainfall forming a wide floodplain that dries up during the summer period, which is typically hot and dry in the Mediterranean region. The flooding cycle starts in September and usually reaches maximum flooding levels (average annual max depth: 0.51 m) at the end of the boreal winter (Díaz-Delgado et al. 2016). Precipitation and groundwater generate small bodies of water (60% of Doñana temporary ponds have a total size \(\ge\) 150 m2), which exhibit inundation periods of variable duration (Gómez-Rodríguez et al. 2009). The northernmost half of the reserve has a greater number of ephemeral ponds than the southern half (‘Las Marismillas’ area). In the south, there are practically no natural ponds and the main water bodies are the ‘zacallones’, which do not dry out (see Fig. 1). Although the average distance between ponds is higher in the North region (North-area, average: 7.2, range 5.9–12.3 km; South-area, average: 4.8, range 3.5–8.0 km), these are likely to show higher connectivity due to favorable in-between (matrix) habitat conditions -puddled meadows-, whereas in the southern edge the surrounding habitat -dry brushwood- could act as a dispersal barrier.
Schematic representation of the main study area, the Doñana National Park (Andalusia, SW Spain), and contrasting landscape features. Photographs illustrate the two types of ponds/habitat types included in the study: (1) temporal lagoons surrounded by meadows that flood recurrently in the North section of the reserve, and (2) ponds (‘zacallones’) that hold water for the whole year and are surrounded by pine woodland in the South part
Sampling
Capture of adult and juvenile newts for DNA sampling was conducted during the breeding season (November–February) in two periods, between 2008 and 2009, and between 2013 and 2015 using crab trap fishing nets. We collected tissue samples via toe-clip from a total of 2733 individuals, which were sampled in 58 ponds (average number of individuals per sampling site: 47.1, range 5–114), 31 from the North region and 27 from the South region (‘zacallones’) (Fig. 1). Additionally, seven populations from Huelva (144 individuals), four populations from Cordoba (23 individuals), one population from Seville (8 individuals), and other one from Badajoz (12 individuals) (Fig. 1b; Fig. S1) were analyzed to compare diversities and genetic distances. Sampling effort (sampling time and number of fishing nets employed) was kept constant across ponds.
All tissues were stored in 95% ethanol and maintained at − 20 °C. Permits for capture and sampling of newts and including ethical considerations, were acquired from the regional authorities.
Genotyping
We extracted approximately 50 μg of genomic DNA of each individual using the QIAGEN DNeasy Tissue Extraction kit. We analyzed thirteen variable microsatellite loci and amplified the fragments as detailed in Albert and Godoy (2011). All PCRs were run with a final volume of 20 μl on an Applied Biosystems ABI 3130xl Genetic Analyzer. Two loci (TpygA8, TpygG140) were dropped after initial testing since these showed a significant deviation from Hardy–Weinberg equilibrium after Bonferroni correction, leaving in all 11 loci which were used for subsequent analysis (Table 1). We also tested for the presence of null alleles using Micro-Checker 2.2.3 (Oosterhout et al. 2004). Alleles were scored using GeneMapper 4.0 (Applied Biosystems) and LIZ 500 size standard.
Genetic diversity
Microsatellite genetic diversity within ponds was quantified by the unbiased estimates of gene diversity: namely, expected heterozygosity (HE), observed heterozygosity (HO), allelic richness (AR) and private allelic richness (PAR, i.e., alleles which are unique to a particular population) using the program GenAlEx 6.5 (Peakall and Smouse 2012). AR and PAR were calculated following the hierarchical rarefaction method available in hp-rare (Kalinowski 2005). Deviations from Hardy–Weinberg equilibrium in each population and for each locus, as well as over all loci, were estimated using an exact probability value. Intra-population subdivision coefficients (FIS) for all sampling sites were computed as an estimate of the level of inbreeding in each pond using the program fstat (Goudet 1995).
Genetic divergence and population structure
Pairwise genetic differentiation was estimated by Weir and Cockerham’s FST (1984) calculated in fstat (Goudet 1995), and the significance of results was evaluated after 15,000 permutations with 95% confidence intervals generated by jack-knifing over loci. FST were computed for each subset (Doñana-North or Doñana-South), separately as well as considering the whole study area (i.e., across the Doñana region). We tested for isolation-by-distance (IBD) by performing a Mantel test of pairwise FST matrix on the log-transformed geographic distance matrix, using the R package ‘ecodist’ (Goslee and Urban 2007) with 9,999 permutations.
Individual population assignment was inferred in GenoDive (Meirmans and Van Tienderen 2004) based on a Monte Carlo test with an \(\alpha\) value of 0.002 on 1000 replicated datasets and 702,811 resampled individuals. We used the recommended \(\alpha\) value of 0.002 (equal to < 1 type I error, sample size*0.002), as a compromise between power to detect migrant and type I errors (Paetkau et al. 2004). Complementarily, we used a Bayesian multilocus genotyping method implemented in the software bayesass 1.3 (Wilson and Rannala 2003) to detect recent gene flow over the last several generations among the four groups identified by structure (see Results). Two runs of 10,000,000 generations (with a burn-in period of 500,000 states) were conducted with all other parameters set to default. This method has been shown to perform well for low migration rates (< 33% of migrant individuals per generation) and under moderate genetic differentiation (Faubet et al. 2007).
We applied a model-based Bayesian clustering approach using the software structure (Pritchard Lab, Stanford University, CA, USA, version 2.3.4) to infer genetic structure and define the number of genetically distinctive populations (i.e., demes) in the dataset. Data were explored using the admixture model and sampling locations as priors, which are considered most appropriate for detecting structure among populations that are likely to be similar due to migration or shared ancestry. Parameters were set as follows: five replicates for each possible number of clusters (K) where (K ranged from 1 to n + 1 for each dataset of n populations) with a burn-in of 100,000 steps followed by 100,000 Markov chain-Monte Carlo (MCMC) iterations. Subsequently, given that the most likely number of genetic clusters inferred in these analyses was always largely below K = 10 (see Results), we performed five additional runs for a reduced range of K with the following settings: 500,000 MCMC iterations with a burn-in period set to 200,000. The optimum number of distinct clusters was estimated with both: the Evanno’s ∆K method (Evanno et al. 2005) implemented in structure harvester (Earl and vonHoldt 2012) and the mean likelihood of the data (mean Ln Pr(\({\rm X}\)|K); Pritchard et al. 2009). Janes et al. (2017) recommended using both methods in tandem since ΔK does not allow the assessment of K = 1 as a potential solution and could overestimate the number of genetic clusters. Optimal alignment of replicates for the same K was obtained for the most relevant K values, and performed in the software clumpp using the ‘greedy’ algorithm. (Jakobsson and Rosenberg 2007). The level of admixture in each population was displayed graphically with distruct (Rosenberg 2004). structure analyses were carried out using four different datasets; one considering all sampled populations in Doñana, other two for each region (Doñana-North; Doñana-South) separately, and another considering 14 randomly selected populations from Doñana (7 from the North and 7 from the South region) and those sampled elsewhere.
Results
Genetic diversity
Ponds in the North region harbor more individuals than ‘zacallones’ from the southern part (average N: 57.8 vs. 34.7, anova; F1,56 = 9.73, p < 0.05). Genetic diversity parameters for each population based on allelic frequencies are summarized in Table 1. The results indicate that northern populations (temporary ponds) possess higher genetic diversity than those located in the South area (‘zacallones’). In the North area, levels of observed heterozygosity (HO) ranged from 0.690 to 0.818 (mean value: 0.742), whereas in the southern populations these values were significantly lower (anova; F1,56 = 21.71, p < 0.001), oscillating between 0.624 and 0.798 (mean value: 0.704). Allelic richness (AR) ranged from 4.1 to 5.4 (mean value: 5.2), and from 3.7 to 4.9 (mean value: 4.5) in the North and South region, respectively (Kruskal–Wallis test; n = 58, H = 37.82, p < 0.05). Doñana populations exhibit higher genetic diversity that populations located at northern latitudes, outside the Doñana region, whose HO values ranged from 0.393 (Badajoz) to 0.737 (Huelva) and their AR was < 3 in all cases. The obtained inbreeding coefficients (FIS) were slightly higher (n = 58, H = 3.10, p = 0.078) within temporary ponds (mean: 0.012, range from − 0.115 to 0.077) in comparison with those observed within the artificially deepened ponds (‘zacallones’) (mean: − 0.010, range from − 0.219 to 0.111). However, that difference decreased (H = 1.80, p = 0.18) after excluding the four northernmost populations, which are more geographically isolated in relation to the remaining populations (see Fig. 2a). Nevertheless, the obtained inbreeding coefficients (FIS) did not differ significantly from zero, suggesting absence of within sampling site substructure or inbreeding. FIS values were substantially higher in populations sampled outside the Doñana wetlands, mostly in populations from Cordoba and Seville (Table 1).
Heatmap plot of FST distances for the pond network located in the North (upper panel) and South (bottom panel) section of the Doñana National Park (see Table 1 for list of localities)
Genetic divergence and population structure
A moderately high level of genetic differentiation was obtained for the dataset including all Doñana populations (overall FST = 0.023). Population differentiation across the whole Doñana region ranged from 0 to 0.097, showing FST values statistically significant (p < 0.05) in 904 of 1613 pairwise comparisons. Differentiation decreased when only northern populations were considered (Doñana-North FST = 0.005, range 0–0.024; Fig. 2), whereas populations from the southern edge show a higher degree of genetic differentiation between them (Doñana-South FST = 0.016, range 0–0.075; Fig. 2). Some of the Doñana populations with the highest average FST values (VET, ENC, HOR, ANG) were among the most geographically isolated populations. We found a significant positive relationship between genetic and geographic distances across the whole Doñana region (Mantel test, r = 0.55, p = 0.001), as well as when both clusters (North and South) were analyzed separately (r = 0.45, p = 0.001 and r = 0.22, p = 0.012, respectively) (Fig. S2). When considering other populations outside Doñana, we observed that FST values were substantially higher (average = 0.158, range 0.04–0.34; Fig. 3). The greatest differentiation was observed between populations located in Badajoz (SER) and Cordoba (CAR, SEQ) and the remaining populations (Huelva and Seville populations).
Heatmap based on FST values among the 15 newt sampling locations in Andalusia (SW Spain). The geographical location of each population is shown in Fig. 4b
The population divergence results also correlated with the results obtained using the Bayesian approach. In the five independent simulations of the Bayesian clustering of individuals method implemented in structure, K = 2 was selected as the most probable number of clusters across the whole National Park, indicating the existence of two well-differentiated zones; Doñana-North (DON) and Doñana-South (DOS) (Fig. 4a). No clear substructure was detected within these clusters when analyzed separately. According to the ∆K method, in the North region of Doñana, the most likely number of clusters present in our dataset was K = 2, yet the estimated log probability of data Pr(X|K) started to plateau (as expected after reaching the best fitting) at K = 1 (Fig. S3). Whilst, in the South region we observed that ∆K peaked at K = 3 probably because there were three populations with distinctive genetic material (VET, ENC, SEG). However, log-likelihood values were similar to those obtained for lower K values (K = 1 and 2), suggesting high levels of admixture (Fig. S4). Our results thus, indicate that the network of ponds in each of the two sectors of the Doñana reserve make up two panmictic populations.
a Location of the sampling sites at the Doñana National Park. The dotted line indicates a dune ridge that separates the North and South regions (green and red color, respectively). The bar graph shows individual assignment probabilities grouped by sampled pond for the two recovered genetic clusters (K = 2). Sites are arranged from North to South. The inset shows the log-likelihood values, Ln Pr (X/K)) and \(\Delta\)K, which indicates the most probable number of genetic clusters for all populations. b Genetic differentiation in the pygmy newt Triturus pygmaeus considering Doñana and other populations from Huelva, Seville, Cordoba, and Badajoz. Admixture proportions at each sampling location of the four genetic clusters recovered by structure are shown in pie-charts. Each individual is represented by a thin line, which is portioned into two segments with a different colour representing the individual’s estimated membership fraction in K cluster. The inset shows an adult individual of T. pygmaeus
When considering Doñana together with other populations from Huelva, Seville, Cordoba and Badajoz provinces, we observed four clusters (Fig. 4b). These clusters corresponded to: Badajoz-Cordoba (EUF, SER, SEQ, CAR), Seville-Huelva North (ARO, BAR, PAD, ARA, CAS), Huelva South including the North section of Doñana (VIL, POR, DON) and Doñana South (DOS). The easternmost population (VEN) showed admixed ancestry instead of high single-cluster assignment. Whereas newt populations from the southern edge of Doñana conform a distinct local cluster (almost exclusively assigned to individuals from Doñana South), the North region of Doñana showed equivalent admixture proportions from clusters associated with the South region of the National Park and nearby populations outside the boundaries of the reserve (southern Huelva) (Fig. 4b). Overall, clusters corresponding to the Northeast populations (Cordoba, Badajoz) were better delimited than those from the Southwest region (Huelva province), which showed less clearly defined cluster boundaries (Fig. S5). Each group included geographically proximate populations.
Contemporary gene flow
bayesass runs yielded low levels of contemporary gene flow among groups (Fig. 5a). The highest migration rates were observed from Group 4 (Doñana South; site: DOS) to Group 3 (Doñana North and Huelva South; sites: DON, POR, VIL), and from Group 1 (Cordoba-Badajoz; sites: SER, EUF, SEQ, CAR) to Group 4. We found evidence of asymmetry in migration between these groups (Fig. 5a). Our results are consistent with the idea that gene flow and dispersal are extremely reduced between groups.
a Estimates of contemporary migration rates based on microsatellite data among the four groups (genetic clusters; see Fig. 4b) of T. pygmaeus. Group 1: SER, EUF, SEQ, VEN, PAD; Group 2: ARO, ARA, BAR, CAR, CAL, CAS; Group 3: DOS, POR, VIL; Group 4: DOS. b Chord diagram of estimated dispersal events according to the assignment test performed in GenoDive. This program quantifies the number of individuals sampled from one putative genetic population that are classified into their population of origin (local individuals) or inferred to belong to another population (immigrant individuals). Sampling locations are given on the outside of the circle. The size of the flow is indicated by the width of the arrow at its base, whose color denotes the donor population
Based on allelic frequencies, the individual population assignment test identified 57 immigrant individuals. Populations from Huelva-South (POR, VIL) showed the highest percentages of migrant individuals (> 25% in both cases), whereas those from CAL and CAS exhibited the highest numbers of non-local (immigrant) individuals (21 and 26%, respectively) (Table S1). Overall, these results suggest that northern populations (Cordoba-Badajoz) are more isolated than those from Seville and Huelva, whose connectivity is less limited (Fig. 5b). Within the Doñana reserve, the North section acts a sink population (immigrants > migrants), whereas the South section acts as donor population (immigrants < migrants), which agrees with the migration rates inferred using bayesass.
Discussion
In this study, we analyzed the genetic diversity and structure of the pygmy newt in a dense network of ponds within the Doñana National Park. Two main types of ponds can be found in this wetland; temporary ponds as a result of the seasonal flood of the marshland, and permanent ponds, artificially deepened for livestock drinking in the driest part of the reserve. Our results suggest that environmental disturbance linked to the regular drying that characterizes temporary ponds does not impact negatively on the genetic variability of populations, which was higher in comparison with that of ponds that do not dry out. According to our findings, pond connectivity may constitute a more important factor than hydroperiod length in determining the genetic diversity and viability of pygmy newt populations in this area. The existence of greater genetic differentiation among ponds (‘zacallones’) in the southern edge suggests that although newts are less time-constrained during their development in this area, they may experience more difficulties in dispersing due to the prevailing habitat, mainly dry brushwood. Whilst, in the North section of Doñana, newts may benefit from a more suitable habitat matrix (wet and muddy meadows), which may facilitate the connectivity among ponds and act as a refuge. However, our findings must be interpreted with caution since, first, we did not quantify the hydroperiod of each pond making it impossible to analyze the relationship between ephemerality and genetic diversity with a considerable sample size, and second, other factors not considered in this study (e.g., differences in other aspects of the environment or landscape) may be responsible for the observed results.
In Doñana, previous authors have reported a reduced body size in this and other amphibian species including Iberian newts Lissotriton boscai, spadefoot toads Pelobates cultripes and natterjack toads Epidalea calamita (e.g., Díaz-Paniagua et al. 1996; Marangoni et al. 2008). Local dwarfism seems to have evolved in response to environmental conditions (dry and warm environments) prevailing in this area (Hyeun-Ji et al. 2020). In unfavorable or unpredictable environments, where adult mortality may be high, newts may increase their specific investment in reproduction at the expense of growth and mature at a younger age. Consequently, individuals from these populations reach sexual maturity earlier, which may lessen the stress associated with the existence of a shorter hydroperiod in most natural ponds. In fact, we did not find smaller populations in the North region, rather the opposite; we observed that temporary ponds harbor more individuals than ‘zacallones’. Our results thus, contrast with those reported by Cayuela et al. (2020a) in yellow-bellied toads Bombina variegata. Cayuela et al. (2020a) showed that B. variegata populations experiencing low-persistence breeding patches had lower genetic variation due to higher emigration rates and longer dispersal distances than did populations utilizing persistent breeding patches despite these showed a denser hydrological network. They concluded that the rate of patch turnover may be a more important driver of neutral genetic variation than landscape connectivity (Cayuela et al. 2020a). The obtained migration rates in each section of the reserve evince asymmetry in migration between these two panmictic populations; we detected that the North section of Doñana receives a higher influx of individuals from the South region than in the opposite direction. Thus, we hypothesize that the effect of ephemerality may be counterbalanced by a weaker landscape resistance, facilitating dispersal among ponds in the North (wetter) region and colonization from the South region of Doñana and other neighboring populations (POR and VIL, Huelva) (Fig. 5b). In addition, we must point out that our pond network constitutes a more predictable environment in comparison with that of Cayuela et al., which consisted of groups of ruts made by logging vehicles that may appear and disappear yearly.
Despite the existence of differences between both regions, the overall level of genetic variability reflects the good status of conservation of Triturus pygmaeus in Doñana. We found moderately high levels of genetic diversity and low inbreeding coefficients revealing that populations of this species, which is locally abundant in this wetland, are not threatened. On the contrary, some pygmy newt populations from northern localities (Cordoba, Badajoz) exhibited lower values of heterozygosity and moderately high (> 0.15) FIS values. These populations belong to natural ponds largely disconnected and, unlike those from Doñana, these do not conform to a network of temporal ponds. Thus, fragmentation has resulted in a loss of genetic diversity, increasing inbreeding processes within these populations, some of which have been listed as ‘Endangered’ and are subject to conservation actions (Reques 2012). It is likely that populations from Eastern Andalusia (Malaga, Granada), the most isolated region, exhibit similar or even lower levels of diversity.
The Bayesian clustering analyses carried out with structure identified two main clusters corresponding to the North and South regions. The two clusters are well-differentiated despite these are only ~ 7 km apart probably due to the existence of a sand dune system preventing the movement of individuals between both regions. The populations included in each cluster had utterly low (~ 0) values of pairwise FST estimates and showed high levels of genetic admixture, especially in the North area (temporary ponds). In the South area (‘Las Marismillas’) a few ‘zacallones’ located in the southern edge (COR, ENC, SEG) were genetically differentiated from the rest. These zacallones could be colonized by individuals from other (not sampled) nearby populations close to the Guadalquivir estuary. Overall, the existence of a higher degree of structuring in the southern edge can be explained by the more intricate habitat existing in this dryer region of the wetland as previously mentioned.
Within each of the two population clusters, we found evidence for isolation-by-distance. The most distinctive ponds in terms of genetic divergence were the most isolated ones (ponds located at the northern and southern edge, respectively). However, excluding the 3–5 ponds located on the periphery of each zone, it seems that dispersal distance does not constitute a limiting factor according to the observed FST values (Fig. 2). It means that geographical distance does not predict genetic differentiation in the core area of each zone. Indeed, the observed average distances between ponds did not exceed the estimated dispersal distances for this or similar species (from 400 to 1500 m; Isselin-Nondedeu et al. 2017). Our findings agree with previous studies on newts in which no genetic structure was found at a local scale (< 15 km) (alpine newt Ichthyosaura alpestris: Prunier et al. 2014; palmate newt Lissotriton helveticus: Isselin-Nondedeu et al. 2017; marbled newt Triturus marmoratus: Costanzi et al. 2018). Thus, the inferred connectivity suggests that pygmy newts do not face significant barriers to dispersal in this region. In this vein, although some other studies of amphibian populations have reported a pattern of isolation by distance and a high degree of genetic structuring (e.g., Kraaijeveld-Smit et al. 2005; Sarasola-Puente et al. 2012; Albert et al. 2015; Mims et al. 2016; Haugen et al. 2020), it is nowadays commonly assumed that when pond networks are separated by these approximate distances (\(\sim\) 10 km), anurans and salamanders are capable to disperse at a rate that make even isolated populations connected to the whole (Smith and Green 2005; Denoël et al. 2018; Cayuela et al. 2020b; Yannic et al. 2021).
When considering other populations outside the Doñana region, we found that the five northermost populations (except VEN) conformed to a distinctive cluster, whereas the eight localities sampled in the easternmost part of Andalusia clustered into another different group. Populations from Cordoba and Badajoz were more strongly differentiated than those from Huelva and Seville, as evidenced by the highest values of pairwise FST values (average FST values: 0.189 vs. 0.139, respectively). These results suggest the existence of moderate genetic differentiation among sampling sites as a result of limited restrictions to genetic exchange across the study region. These restrictions seem to be more pronounced in populations from the northern part, located in Sierra Morena, due to the complex orography of this region in comparison with that of the Guadalquivir River Basin which runs through Huelva and Seville. When analyzing Doñana populations together with those sampled elsewhere, we observed some level of admixture between populations from Huelva (VIL, POR) and populations located within the boundaries of the National Park (North section of Doñana). In light of these results, we speculate that occasional gene flow may have occurred between the populations located in the Guadalquivir flood plain despite the dense road network surrounding the natural areas. Our analysis of contemporary gene flow supports that idea; although we observed that current levels of gene flow among populations are moderately low, it seems that dispersal is less reduced between localities from Huelva (Groups 2 and 3). Overall, our results indicate that drift is prevalent relative to migration in explaining present-day patterns of genetic differentiation.
Conclusions
This is the first study reporting genetic diversity estimates in T. pygmaeus populations, an endemic species of the Iberian Peninsula catalogued as “Endangered” or “Threatened” in some regions of SW Spain (Consejería de Medio Ambiente 2001; Reques 2012). Our results show that pygmy newt populations in Doñana form two-well differentiated clusters with considerable levels of genetic admixture within each of them. We found evidence of asymmetry in migration between these two panmictic populations; Doñana North acts as a recipient group and Doñana South as donor. Although, one might think that artificial (permanent) ponds are beneficial for amphibians and constitute an unequivocally successful measure, this study suggests that its effect largely depends on the surrounding habitat in which these are embedded (see also Joly 2001; Calhoun et al. 2014). Our results agree with previous studies that investigated the interactive effects of pond and landscape variables on amphibian abundance and concluded that both local (e.g., pond permanence) and landscape features are important (van Buskirk 2005; Denoël et al. 2013). Nevertheless, the value of artificial ponds as effective tool to improve the genetic variability at meta-population level must not be underestimated. Our results evince that despite southern populations exhibit lower diversity and higher rates of inbreeding, they seem to be an important source of gene flow for the northern populations. Thus, these human-made ponds (‘zacallones’) probably contribute significantly to the good state of pygmy newt populations as well as other species such as the Iberian newt or the Iberian green frog Pelophylax perezi (Díaz-Paniagua et al. 2007). Whereas Doñana populations are in a non-worrying situation, a genetic screening of more peripheral populations revealed that they exhibit low genetic diversity. It indicates that these newt populations are so isolated that inbreeding and genetic drift are no longer counterbalanced by gene flow. Consequently, conservation actions aimed at protecting this species should focus on improving the connectivity of their more fragmented populations, especially in orographically intricate regions like Sierra Morena (Cordoba) and Cordillera Subbética (Granada-Malaga). This goal seems affordable according to our findings, which reinforce the view that amphibian dispersal is not as uniformly limited as is often thought.
Data availability
All genetics (genotypes) and related information is available upon request to the authors.
References
Albert EM, Godoy JA (2011) Characterization of 13 microsatellite loci for the Pygmy Marbled Newt Triturus pygmaeus (Salamandridae). Conserv Genet Resour 3:745–747
Albert EM, Fernández-Beaskoetxea S, Godoy JA, Tobler U, Schmidt BR, Bosch J (2015) Genetic management of an amphibian population after a chytridiomycosis outbreak. Conserv Genet 16:103–111
Arntzen JW, García-París M (1995) Morphological and allozyme studies of midwife toads (genus Alytes), including the description of two new taxa from Spain. Bijdragen Tot De Dierkunde 65:5–34
Arntzen JW, López-Delgado J, van Riemsdijk I, Wielstra B (2021) A genomic footprint of a moving hybrid zone in marbled newts. J Zool Syst Evol Res 59:459–465
Bagella S, Gascón S, Filigheddu R, Cogoni A, Boix D (2016) Mediterranean temporary ponds: new challenges from a neglected habitat. Hydrobiologia 782:1–10
Boix D, Gascón S, Sala J, Badosa A, Brucet S, López-Flores R, Martinoy M, Gifre J, Quintana X (2008) Patterns of composition and species richness of crustaceans and aquatic insects along environmental gradients in Mediterranean water bodies. Hydrobiologia 597:53–69
Brannelly LA, Ohmer MEB, Saenz V, Richards-Zawacki CL (2019) Effects of hydroperiod on growth, development, survival and immune defences in a temperate amphibian. Funct Ecol 33:1952–1961
Calhoun AJK, Arrigoni J, Brooks RP, Hunter ML, Richter SC (2014) Creating successful vernal pools: a literature review and advice for practitioners. Wetlands 34:1027–1038
Cayuela H, Besnard A, Cote J, Laporte M, Bonnaire E, Pichenot J (2020a) Anthropogenic disturbance drives dispersal syndromes, demography, and gene flow in amphibian populations. Ecol Monogr 90:e01406
Cayuela H, Valenzuela-Sánchez A, Teulier L, Martínez-Solano I (2020b) Determinants and consequences of dispersal in vertebrates with complex life cycles: a review of pond-breeding amphibians. Q Rev Biol 95:1–36
Cimmaruta R, Scialanca F, Luccioli F, Nascetti G (2003) Genetic diversity and environmental stress in Italian populations of the cyprinodont fish Aphanius fasciatus. Oceanol Acta 26:101–110
Collinson NH, Biggs J, Corfield A, Hodson MJ, Walker D, Whitfield M, Williams PJ (1995) Temporary and permanent ponds: an assessment of the effects of drying out on the conservation value of aquatic macroinvertebrate communities. Biol Conserv 74:123–133
Consejería de Medio Ambiente (2001) Libro Rojo de los Vertebrados Amenazados de Andalucía. Junta de Andalucía, 336 pp.
Costanzi J-M, Mège P, Boissinot A, Isselin-Nondedeu F, Guérin S, Picard D (2018) Agricultural landscapes and the Loire River influence the genetic structure of the marbled newt in Western France. Sci Rep 8:14177
Denoël M, Perez A, Cornet Y, Ficetola GF (2013) Similar local and landscape processes affect both a common and a rare newt species. PLoS ONE 8:e62727
Denoël M, Dalleur S, Langrand E, Besnard A, Cayuela H (2018) Dispersal and alternative breeding site fidelity strategies in an amphibian. Ecography 41:1543–1555
Díaz-Delgado R, Aragonés D, Afán I, Bustamante J (2016) Long-term monitoring of the flooding regime and hydroperiod of doñana marshes with landsat time series (1974–2014). Remote Sens 8:775
Díaz-Paniagua C (1990) Temporary ponds as breeding sites of amphibians at a locality in Southwestern Spain. Herpetol J 1:447–453
Díaz-Paniagua C, Mateo JA, Andreu AC (1996) Age and size structure of populations of small marbled newts (Triturus marmoratus pygmaeus) from Doñana National Park (SW Spain). A case of dwarfism among dwarfs. J Zool 239:83–92
Díaz-Paniagua C, Gómez-Rodríguez C, Portheault A, de Vries W (2005) Los Anfibios de Doñana. Organismo Autónomo de Parques Nacionales. Ministerio de Medio Ambiente, Madrid
Díaz-Paniagua C, Gómez-Rodríguez C, Portheault A, de Vries W (2007) Distribución de los anfibios del Parque Nacional de Doñana en función de la densidad y abundancia de los hábitats de reproducción. Revista Española De Herpetología 20:17–30
Díaz-Paniagua C, Fernández-Zamudio R, Florencio M, García-Murillo P, Gómez-Rodríguez C, Siljeström P et al (2010) Temporary ponds from the Doñana National Park: a system of natural habitats for the preservation of aquatic flora and fauna. Limnetica 29:41–58
Díaz-Paniagua C, Fernández-Zamudio R, Serrano L, Florencio M (2016) El sistema de lagunas temporales del Parque Nacional deDoñana: aplicación a la gestión y conservación de hábitats acuáticos singulares. Proyectos de Investigación en Parques Nacionales2011-2014. Colección Investigación en la Red de Parques Nacionales. Organismo Autónomo de Parques Nacionales. Ministerio deMedio Ambiente, Madrid, pp 37–59
Earl DA, vonHoldt BM (2012) Structure harvester: a website and program for visualizing structure output and implementing the Evanno method. Conserv Genet Res 4:359–361
Espregueira Themudo G (2010) Newts in time and space: the evolutionary history of Triturus newts at different temporal and spatial scales. Thesis dissertation. Leiden University, Netherlands
Espregueira Themudo G, Arntzen JW (2007) Newts under siege: range expansion of Triturus pygmaeus isolates populations of its sister species. Divers Distrib 13:580–586
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software structure: a simulation study. Mol Ecol 14:2611–2620
Faubet P, Waples RS, Gaggiotti OE (2007) Evaluating the performance of a multilocus Bayesian method for the estimation of migration rates. Mol Ecol 16:1149–1166
Fernández-Delgado C (2017) Doñana natural space: the uncertain future of a crown jewel in Europe’s protected areas. Case Stud Environ 1:23–112
Florencio M, Serrano L, Gómez-Rodríguez C, Millán A, Díaz-Paniagua C (2009) Inter and intra-annual variations of macroinvertebrate assemblages are related to the hydroperiod in Mediterranean temporary ponds. Hydrobiologia 634:167–183
Fox CW, Reed DH (2011) Inbreeding depression increases with environmental stress: an experimental study and meta-analysis. Evolution 65:246–258
García-París M (2002) Triturus pygmaeus. In: Pleguezuelos JM, Márquez R, Lizana L (eds) Atlas y Libro Rojo de los Anfibios y Reptiles de España. Dirección General de Conservación de la Naturaleza-Asociación Herpetológica Española, Madrid, pp 70–72
García-París M, Arano B, Herrero P (2001) Molecular characterization of the contact zone between Triturus pygmaeus and T. marmoratus (Caudata: Salamandridae) in Central Spain and their taxonomic assessment. Revista Española De Herpetología 15:115–126
Gómez-Rodríguez C, Díaz-Paniagua C, Serrano L, Florencio M, Portheault A (2009) Mediterranean temporary ponds as amphibian breeding habitats: the importance of preserving pond networks. Aquat Ecol 43:1179–1191
Gómez-Rodríguez C, Díaz-Paniagua C, Bustamante J (2011) Cartografía de las lagunas temporales del Parque Nacional de Doñana. Agencia Andaluza del Agua, Consejería de Medio Ambiente, Junta de Andalucía
Goslee SC, Urban DL (2007) The ecodist package for dissimilarity-based analysis of ecological data. J Stat Softw 22:1–19
Goudet J (1995) FSTAT (version 1.2): a computer program to calculate F-statistics. J Hered 86:485–486
Green AJ, Bustamante J, Janss GFE, Fernández-Zamudio R, Díaz-Paniagua C (2018) Doñana wetlands (Spain). In: Finlayson CM, Milton GR, Prentice RC, Davidson NC (eds) The wetland book: II: distribution, description and conservation. Springer, New York, pp 1123–1136
Haugen H, Linløkken A, Østbye K, Heggenes J (2020) Landscape genetics of northern crested newt Triturus cristatus populations in a contrasting natural and human-impacted boreal forest. Conserv Genet 21:515–530
Hyeun-Ji L, Broggi J, Sánchez-Montes G, Díaz-Paniagua C, Gomez-Mestre I (2020) Dwarfism in close continental amphibian populations despite lack of genetic isolation. Oikos 129:1243–1256
Isselin-Nondedeu F, Trochet A, Joubin T, Picard D, Etienne R, Le Chevalier H, Legrand D, Ribéron A (2017) Spatial genetic structure of Lissotriton helveticus L. following the restoration of a forest ponds network. Conserv Genet 18:853–866
IUCN (2021) IUCN Red List of Threatened Species. Version 2021. http://www.iucnredlist.org.
Jakobsson M, Rosenberg NA (2007) CLUMPP: a cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics 23:1801–1806
Janes JK, Miller JM, Dupuis JR et al (2017) The K = 2 conundrum. Mol Ecol 26:3594–3602
Joly P, Miaud C, Lehmann A, Grolet O (2001) Habitat matrix effects on pond occupancy in newts. Conserv Biol 15:239–248
Kalinowski ST (2005) HP-RARE 1.0: A computer program for performing rarefaction on measures of allelic richness. Mol Ecol Resour 5:187–189
Keller LF, Waller DM (2022) Inbreeding effects in wild populations. Trends Ecol Evol 17:230–241
Kraaijeveld-Smit FJL, Beebee TJC, Griffiths RA, Moore RD, Schley L (2005) Low gene flow but high genetic diversity in the threatened Mallorcan midwife toad Alytes muletensis. Mol Ecol 14:3307–3315
Lesbarrères D, Primmer CR, Laurila A, Merilä J (2005) Environmental and population dependency of genetic variability-fitness correlations in Rana temporaria. Mol Ecol 14:311–323
Marangoni F, Gómez-Mestre I, Tejedo M (2008) Extreme reduction in body size and reproductive output associated with sandy substrate in two anuran species. Amphibia-Reptilia 29:541–553
Meirmans PG, Van Tienderen PH (2004) Genotype and Genodive: two programs for the analysis of genetic diversity of asexual organisms. Mol Ecol Notes 4:792–794
Mims MC, Hauser L, Goldberg CS, Olden JD (2016) Genetic differentiation, isolation-by-distance, and metapopulation dynamics of the Arizona treefrog (Hyla wrightorum) in an isolated portion of its range. PLoS ONE 11:e0160655
Nicolet P, Biggs J, Fox G, Hodson MJ, Reynolds C, Whitfield M, William PJ (2004) The wetland plant and macroinvertebrate assemblages of temporary ponds in England and Wales. Biol Conserv 120:265–282
Oosterhout CV, Hutchinson WF, Wills DPM, Shipley P (2004) Micro-Checker: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4:535–538
Paetkau D, Slade R, Burden M, Estoup A (2004) Genetic assignment methods for the direct, real-time estimation of migration rate: a simulation-based exploration of accuracy and power. Mol Ecol 13:55–65
Peakall R, Smouse PE (2012) GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics 28:2537–2539
Pritchard JK, Wen X, Falush D (2009) Documentation for STRUCTURE software
Prunier JG, Kaufmann B, Léna JP, Fenet S, Pompanon F, Joly P (2014) A 40-year-old divided highway does not prevent gene flow in the alpine newt Ichthyosaura alpestris. Conserv Genet 15:453–468
Reques R, Pleguezuelos JM, Caro J (2006) Parajes de Interés Herpetológico en Andalucía. Informe técnico de la Consejería de Medio Ambiente. Junta De Andalucía I–II:572
Reques, R. (2012) Programa de actuaciones para la conservación de los anfibios amenazados de Andalucía, Fase II. Informe Final. European Union-Junta de Andalucía. Consejería de Medio Ambiente.
Ribeiro R, Carretero MA, Sillero N, Alarcos G, Ortiz-Santaliestra M, Lizana M, Llorente GA (2011) The pond network: can structural connectivity reflect on (amphibian) biodiversity patterns? Landsc Ecol 26:673–682
Rosenberg NA (2004) DISTRUCT: a program for the graphical display of population structure. Mol Ecol Resour 4:137–138
Sarasola-Puente V, Madeira MJ, Gosá A, Lizana M, Gómez-Moliner B (2012) Population structure and genetic diversity of Rana dalmatina in the Iberian Peninsula. Conserv Genet 13:197–209
Semlitsch RD (1996) Structure and dynamics of an amphibian community. In: Cody ML, Smallwood JL (eds) Long-term studies of vertebrate communities. Academic Press, London, pp 217–248
Semlitsch RD, Peterman WE, Anderson TL, Drake DL, Ousterhout BH (2015) Intermediate pond sizes contain the highest density, richness, and diversity of pond-breeding amphibians. PLoS ONE 10:e0123055
Sequeira F, Bessa-Silva A, Tarroso P, Sousa-Neves T, Vallinoto M, Gonçalves H, Martínez-Solano I (2020) Discordant patterns of introgression across a narrow hybrid zone between two cryptic lineages of an Iberian endemic newt. J Evol Biol 33:202–216
Serrano L, Reina M, Martín G, Reyes I, Arechederra A, León D, Toja J (2006) The aquatic systems of Doñana (SW Spain): watersheds and frontiers. Limnetica 25:11–32
Smith MA, Green DM (2005) Dispersal and the metapopulation paradigm in amphibian ecology and conservation: are all amphibian populations metapopulations? Ecography 28:110–128
Tejedo M, Marangoni F, Pertoldi C, Richter-Boix A, Laurila A, Orizaola G (2010) Contrasting effects of environmental factors during larval stage on morphological plasticity in post-metamorphic frogs. Climate Res 43:31–39
Torres JM, Hernández I, Reques R (2015) Local and landscape influence on richness of amphibian species breeding in seasonal ponds in the Spanish south-Atlantic littoral Impact Determination. Basic Appl Herpetol 29:5–19
Tournier E, Besnard A, Tournier V, Cayuela H (2017) Manipulating waterbody hydroperiod affects movement behaviour and occupancy dynamics in an amphibian. Freshw Biol 62:1768–1782
Van Buskirk J (2005) Local and landscape influence on amphibian occurrence and abundance. Ecology 86:1936–1947
Wang IJ, Johnson JR, Johnson BB, Shaffer HB (2011) Effective population size is strongly correlated with breeding pond size in the endangered California tiger salamander, Ambystoma californiense. Conserv Genet 12:911–920
Watts AG, Schlichting PE, Billerman SM, Jesmer BR, Micheletti S, Fortin M-J, Funk WC, Hapeman P, Muths E, Murphy MA (2015) How spatio-temporal habitat connectivity affects amphibian genetic structure. Front Genet 6:275
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population-structure. Evolution 38:1358–1370
Wilson GA, Rannala B (2003) Bayesian inference of recent migration rates using multilocus genotypes. Genetics 163:1177–1191
Yannic G, Helfer V, Sermier R, Schmidt BR, Fumagalli L (2021) Fine scale genetic structure in fire salamanders (Salamandra salamandra) along a rural-to-urban gradient. Conserv Genet 22:275–292
Acknowledgements
Many people helped during the field work or provided samples: Pim Arntzen, Eva Aylagas, Maricel Aguilar, David Daversa, Carlos Marfil, David Ragel, Isidro Román and the staff of Doñana National Park and the monitoring research team of the Reserva Biológica de Doñana. We thank Isabel Máximo, Juanmi Arroyo and Conchi Cáliz for their help in the lab. Alessandro Vindigni (University of Zurich) provided technical support and Isabel Afán (LAST-EBD) kindly provided the maps shown in Figs. 1 and 4. This study is dedicated to the memory of evolutionary ecologist and herpetologist Josh Van Buskirk (1959-2021) who passed away after a long battle against cancer.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This study was funded by the grants of the Government of Andalusia (Consejería de Economía, Innovación y Ciencia) through the projects P07-RNM-02928 and RNM-8147. V. García-Navas was supported by a “Ramón y Cajal” contract (ref. RYC2019-026703-I) from the Spanish Ministry of Science and Innovation.
Author information
Authors and Affiliations
Contributions
EA and VGN designed the study and analyzed the data. EA collected the data and performed the labwork. VGN wrote the first draft of manuscript assisted by EA.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest or competing interests.
Consent to participate
All authors provided informed consent to participate to the present work.
Consent to publish
All authors provided informed consent to publish the present work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Albert, E.M., García-Navas, V. Population structure and genetic diversity of the threatened pygmy newt Triturus pygmaeus in a network of natural and artificial ponds. Conserv Genet 23, 575–588 (2022). https://doi.org/10.1007/s10592-022-01437-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-022-01437-7