Abstract
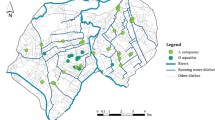
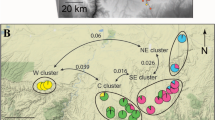
Allegheny woodrats (Neotoma magister) exist as groups of metapopulations due to their dependence on naturally disjunct rocky outcrops in the eastern United States. Severe demographic declines of Allegheny woodrats have occurred in many parts of the range due to a myriad of interacting processes, therefore identifying factors that help maintain the integrity of metapopulations is needed to guide conservation efforts. One factor considered critical to metapopulation persistence is maintaining functional connectivity. Therefore, our objective was to identify landscape factors that influence gene flow in Allegheny woodrats. We sampled 159 individuals from throughout the distribution in Virginia, genotyped them at 22 microsatellite loci, and modeled effects of land cover, roads, large rivers, and elevation on gene flow. The model that parameterized areas ≤ 750 m in elevation as a barrier to gene flow performed best among the tested models, which indicates that functional connectivity is highest in high elevation habitats. We found little evidence for anthropogenic factors influencing gene flow, but continued study across extant metapopulations is needed in more peripheral areas to evaluate if anthropogenic barriers impact functional connectivity. Overall, our study emphasizes the importance of considering elevation for maintaining functional connectivity of Allegheny woodrats in the face of ongoing demographic declines in the eastern United States.


Similar content being viewed by others
References
Adams RV, Burg TM (2015) Gene flow in a forest-dependent bird across a fragmented landscape. PLoS ONE 10(11):e0140938. https://doi.org/10.1371/journal.pone.0140938
Alacs EA, Janzen FJ, Scribner KT (2007) Genetic issues in freshwater turtle and tortoise conservation. In: Shaffer HB, FitzSimmons NN, Georges A, Rhodin AGJ (eds) Defining turtle diversity: proceedings of a workshop on genetics, ethics and taxonomy of freshwater turtles and tortoises, vol 4. Chelonian Research Monographs, pp 107–123
Anderson CD, Epperson BK, Fortin M, Holderegger R, James PMA, Rosenberg MS, Scribner KT, Spear S (2010) Considering spatial and temporal scale in landscape-genetic studies of gene flow. Mol Ecol 19:3565–3575
Anderson SJ, Kierepka EM, Swihart RK, Latch EK, Rhodes OE Jr (2015) Assessing the permeability of landscape features to animal movement: using genetic structure to infer functional connectivity. PLoS ONE 10(2):e0117500. https://doi.org/10.1371/journal.pone.0117500
Baguette M (2003) Long distance dispersal and landscape occupancy in a metapopulation of the cranberry fritillary butterfly. Ecography 26:153–160
Balcom BJ, Yahner RH (1996) Microhabitat and landscape characteristics associated with the threatened Allegheny woodrat. Conserv Biol 10:515–523
Bartón K (2016) R Package MuMIn. https://cran.r-project.org/web/packages/MuMIn/index.html
Beatty WS, Beasley JC, Dharmarajan G, Rhodes OE Jr (2012) Genetic structure of a Virginia opossum (Didelphis virginiana) population inhabiting a fragmented agricultural system. Can J Zool 90:101–109
Benjamini Y, Yekutieli D (2005) False discovery rate-adjusted multiple confidence intervals for selected parameters. J Am Stat Assoc 100:71–81
Birch G, Feldhamer G, Dyer W (1994) Helminths of the gastrointestinal tract of raccoons in southern Illinois with management implications of Baylisascaris procyonis occurrence. Trans Ill Acad Sci 87:165–170
Bohonak AJ (1999) Dispersal, gene flow, and population structure. Q Rev Biol 74:21–45
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer-Verlag, New York
Castillo JA, Epps CW, Davis AR, Cushman SA (2014) Landscape effects on gene flow for a climate-sensitive montane species, the American pika. Mol Ecol 23:843–856
Castleberry SB (2008) Home range, movements, and habitat selection. In: Peles JD, Wright J (eds) The Allegheny woodrat: ecology, conservation, and management of a declining species. Springer, New York, pp 63–73
Castleberry SB, King TL, Wood PB et al (2000) Microsatellite DNA markers for the study of Allegheny woodrat (Neotoma magister) populations and cross-species amplification in the genus Neotoma. Mol Ecol 9:824–826
Castleberry SB, Ford WM, Wood PB et al (2001) Movements of Allegheny woodrats in relation to timber harvesting. J Wildl Manag 65:148–156
Castleberry SB, King TL, Wood PB et al (2002) Microsatellite DNA analysis of population structure in Allegheny woodrats (Neotoma magister). J Mammal 83:1058–1070
Castleberry SB, Mengak MT, Ford WM (2006) Neotoma magister. Mamm Species 789:1–5
Chamblin HD, Wood PB, Edwards JW (2004) Allegheny woodrat (Neotoma magister) use of rock drainage channels on reclaimed mines in southern West Virginia. Am Mid Nat 151:346–354
Clarke RT, Rothery P, Raybould AF (2002) Confidence limits for regression relationships between distance matrices: estimating gene flow with distance. J Agric Biol Environ Stat 7:361–372
Cushman SA, McKelvey KS, Hayden J et al (2006) Gene flow in complex landscapes: testing multiple hypotheses with causal modeling. Am Nat 168:486–499
Cushman SA, Shirk A, Languth EL (2012) Separating the effects of habitat area, fragmentation and matrix resistance on genetic differentiation in complex landscapes. Landsc Ecol 27:369–380
Don RH, Cox PT, Wainright BJ et al (1991) ‘Touchdown’ PCR to circumvent spurious priming during gene amplification. Nucleic Acids Res 19:4008
Edwards SV (1993) Long-distance gene flow in a cooperative breeder detected in genealogies of mitochondrial DNA sequences. Proc R Soc Lond B 252:177–185
Ford WM, Castleberry SB, Mengak MT et al (2006) Persistence of Allegheny woodrats (Neotoma magister) across the mid-Atlantic Appalachian Highlands landscape, USA. Ecography 29:745–754
Gannon WL, Sikes RS (2007) Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J Mammal 88:809–823
Gauffre B, Estoup A, Bretagnolle V, Cosson JF (2008) Spatial genetic structure of a small rodent in a heterogeneous landscape. Mol Ecol 17:4619–4629
Gesch DB (2007) The national elevation dataset. In: Maune D (ed) Digital elevation model technologies and applications: the DEM users manual, 2nd edn. American Society for Photogrammetry and Remote Sensing, Bethesda, pp 99–118
Gesch DB, Oimoen M, Greenlee S et al (2002) The national elevation dataset. Photogramm Eng Rem Sens 68:5–11
Hanski I (1997) Metapopulation dynamics: from concepts and observations to predictive models. In: Hanski I, Gilpin M (eds) Metapopulation biology; ecology, genetics and evolution. Academic Press, London, pp 69–91
Hardy OJ, Vekemans X (2002) SPAGeDi: a versatile computer program to analyse spatial genetic structure at the individual or population levels. Mol Ecol Notes 2:618–620
Kanine JM (2013) Conservation and landscape genetics of Allegheny woodrats (Neotoma magister) in Virginia. Dissertation, University of Georgia, Athens, GA, USA
Keenan K, McGinnity P, Cross TF, Crozier WW, Prodöhl PA (2013) diveRsity: an R package for the estimation and exploration of population genetics parameters and their associated errors. Methods Ecol Evol 4:782–788
Kibble WA (2007) OligoCalc: an online oligonucleotide properties calculator. Nucleic Acids Res 35:W43–W46
Kierepka EM, Latch EK (2016) Fine-scale landscape genetics of the American Badger (Taxidea taxus): disentangling landscape effects and sampling artifacts in a poorly understood species. Heredity 116:33–43
Kierepka EM, Anderson SJ, Swihart RK, Rhodes OE Jr (2016) Evaluating the influence of life-history characteristics on genetic structure: a comparison of small mammals inhabiting complex agricultural landscapes. Ecol Evol 6:6376–6396
Kinlan BP, Gaines SD (2003) Propagule dispersal in marine and terrestrial environments: a community perspective. Ecology 84:2007–2020
Koenig WD, VanVuren D, Hooge PN (1996) Detectability, philopatry, and the distribution of dispersal distances in vertebrates. Trends Ecol Evol 11:514–517
Latch EK, Rhodes OE Jr (2005) The effects of gene flow and population isolation on the genetic structure of introduced wild turkey populations: are genetic signatures of source populations retained. Conserv Genet 6:981–997
LoGiudice K (2003) Trophically transmitted parasites and the conservation of small populations: raccoon roundworm and the imperiled Allegheny woodrat. Conserv Biol 17:258–266
LoGiudice K (2006) Toward a synthetic view of extinction: a history lesson from a North American rodent. Bioscience 56:687–693
Lowe WH, Allendorf FW (2010) What can genetics tell us about population connectivity? Mol Ecol 14:3219–3234
Manel S, Schwartz MK, Luikart G, Taberlet P (2003) Landscape genetics: combining landscape ecology and population genetics. Trends Ecol Evol 18:189–197
Manjerovic ME (2004) Demography and genetic structure of an Allegheny woodrat population in northcentral West Virginia. Thesis, West Virginia University, Morgantown, West Virginia, USA
Manjerovic MB, Wood PB, Edwards JW (2009) Mast and weather influence on population trends of a species of concern: the Allegheny woodrat. Am Mid Nat 162:52–61
Matocq MD (2001) Characterization of microsatellite loci in the dusky-footed woodrat Neotoma fuscipes. Mol Ecol Notes 1:194–196
McGowan E (1993) Experimental release and fate study of the Allegheny woodrat (Neotoma magister). In: Federal aid project report W-166-E-1. New York Department of Environmental Conservation, Delmar, New York
McRae BH, Beier P (2007) Circuit theory predicts gene flow in plant and animal populations. Proc Natl Acad Sci USA 104:19885–19890
McRae BH, Dickson BG, Keitt TH et al (2008) Using circuit to model connectivity in ecology, evolution, and conservation. Ecology 89:2712–2724
Mengak MT (2002) Analysis and summary of eleven years of Allegheny woodrat trapping data in southwest Virginia, 1990–2000. Final report submitted to Virginia Department of Game and Inland Fisheries, Richmond, Virginia
Merriam G, Kozakiewiez M, Tsuchiya E, Hawley K (1989) Barriers as boundaries for metapopulations and demes of Peromyscus leucopus in farm landscapes. Landsc Ecol 2:227–235
Micheletti SJ, Storfer A (2015) A test of the central–marginal hypothesis using population genetics and ecological niche modelling in an endemic salamander (Ambystoma barbouri). Mol Ecol 24:967–979
Munshi-South J, Kharchenko K (2010) Rapid, pervasive genetic differentiation of urban white-footed (Peromyscus leucopus) populations in New York City. Mol Ecol 19:4242–4254
Myers RT (1997) Microhabitat and ecology of the Allegheny woodrat in northcentral West Virginia. Thesis, West Virginia University, Morgantown, West Virginia
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142
Ray DK (2000) Phylogenetics and evolution of woodrats (genus Neotoma) in the southern Appalachian Mountains. Thesis, University of North Carolina Wilmington, Wilmington, North Carolina
Richardson JL, Brady SP, Wang IJ, Spear SF (2016) Navigating the pitfalls and promise of landscape genetics. Mol Ecol 25:849–863
Rico A, Kindlmann P, Sedláček F (2009) Can the barrier effect of highways cause genetic subdivision in small mammals. Acta Theriol 54:297–310
Rousset F (2000) Genetic differentiation between individuals. J Evol Biol 13:58–62
Row JR, Knick ST, Oyler-McCance SJ, Lougheed SC, Fedy BC (2016) Developing approaches for linear mixed modeling in landscape genetics through landscape-directed dispersal simulations. Ecol Evol 7:3751–3761
Short Bull RA, Cushman SA, Mace R, Chilton T, Kendall KC, Landguth EL, Schwartz MK, McKelvey K, Allendorf F, Luikart G (2011) Why replication is important in landscape genetics: American black bear in the Rocky Mountains. Mol Ecol 20:1092–1107
Slatkin M (1987) Gene flow and the geographic structure of natural populations. Science 236:787–792
Smyser TJ, Rhodes OE Jr (2008) Genetic diversity of woodrats: implications for conservation. In: Peles JD, Wright J (eds) The Allegheny woodrat: ecology, conservation, and management of a declining species. Springer, New York, pp 153–167
Smyser TJ, Duchamp JE, Johnson SA, Larkin JL, Rhodes OE Jr (2012a) Consequences of metapopulation collapse: comparison of genetic attributes between two Allegheny woodrat metapopulations. Conserv Genet 13:849–858
Smyser TJ, Johnson SA, Page LK, Rhodes OE Jr (2012b) Synergistic stressors and the dilemma of conservation in a multivariate world: a case study in Allegheny woodrats. Anim Conserv 15:205–213
Smyser TJ, Johnson SA, Page LK, Hudson CM, Rhodes OE Jr (2013) Use of experimental translocations of Allegheny woodrat to decipher causal agents of decline. Conserv Biol 27:752–762
Smyser TJ, Stauffer GE, Johnson SA, Hudson CM, Rhodes OE Jr, Swihart RK (2016) Annual survival of Allegheny woodrats in a nonequilibrium metapopulation. J Mammal 97:1699–1708
Sousa B, Svensson E, Maria L et al (2007) Characterization of 18 microsatellite loci for woodrats of the Neotoma lepida group (Rodentia, Cricetidae, Neotominae). Mol Ecol Notes 7:868–870
Swihart RK, Slade NA (1984) Road crossing in Sigmodon hispidus and Microtus ochrogaster. J Mammal 65:357–360
Trakhtenbrot A, Nathan R, Perry G, Richardson DM (2005) The importance of long-distance dispersal in biodiversity conservation. Divers Distrib 11:173–181
Van Strien MJ, Keller DA, Holderegger R (2012) A new analytical approach to landscape genetic modelling: least-cost transect analysis and linear mixed models. Mol Ecol 21:4010–4023
Wagner V, Durka W, Hensen I (2011) Increased genetic differentiation but no reduced genetic diversity in peripheral vs. central populations of a steppe grass. Am J Bot 98:1173–1179
Wood PB (2008) Woodrat population dynamics and movement patterns. In: Peles JD, Wright J (eds) The Allegheny woodrat: ecology, conservation, and management of a declining species. Springer, New York, pp 45–62
Wright S (1943) Isolation by distance. Genetics 28:114–138
Wright J (2008) History and current status of the Allegheny woodrat. In: Peles JD, Wright J (eds) The Allegheny woodrat: ecology, conservation, and management of a declining species. Springer, New York, pp 3–22
Wright J, Kirkland GL (2000) A possible role for chestnut blight in the decline of the Allegheny woodrat. J Am Chestnut Found 8:30–35
Acknowledgements
Funding was provided by the Virginia Department of Game and Inland Fisheries (VDGIF) (Grant No. 2009-11559), the Virginia Academy of Science, and the Warnell School of Forestry and Natural Resources at the University of Georgia. Housing was secured throughout the project with the assistance of VDGIF, national park, and national forest biologists and staff. We thank T. Menken, D. Sollenberger, E. Glancy, L. Mengak, K. Phillips, R. Reynolds, A. Burgeois, F. Frenzel, C. Croy, E. Haverlack, J. Overcash, L. Boggs, R. Gubler, and J. Beeler for assistance with field work and project planning.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kanine, J.M., Kierepka, E.M., Castleberry, S.B. et al. Influence of landscape heterogeneity on the functional connectivity of Allegheny woodrats (Neotoma magister) in Virginia. Conserv Genet 19, 1259–1268 (2018). https://doi.org/10.1007/s10592-018-1093-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-018-1093-4