Abstract
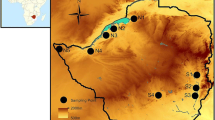
Understanding patterns of genetic diversity and population structure for rare, narrowly endemic plant species, such as Pinguicula ionantha (Godfrey’s butterwort; Lentibulariaceae), informs conservation goals and can directly affect management decisions. Pinguicula ionantha is a federally listed species endemic to the Florida Panhandle in the southeastern USA. The main goal of our study was to assess patterns of genetic diversity and structure in 17 P. ionantha populations, and to determine if diversity is associated with geographic location or population characteristics. We scored 240 individuals at a total of 899 AFLP markers (893 polymorphic markers). We found no relationship between the estimated population size with either of two measures of diversity (proportion of loci polymorphic, P = 0.37; Nei’s gene diversity, P = 0.50). We also found low levels of population genetic structure; there was no clear relationship of genetic isolation by distance (P = 0.23) and only a small (but significant) proportion of genetic variation was partitioned amongst regions (2.4 %, P = 0.02) or populations (20.8 %, P < 0.001). STRUCTURE analysis found that the model with two inferred clusters (K = 2) best described the AFLP data; the dominant cluster at each site corresponded to the results from PCoA and Nei’s genetic distance analyses. The observed patterns of genetic diversity suggest that although P. ionantha populations are isolated spatially by distance and both natural and anthropogenic barriers, some gene flow occurs among them or isolation has been too recent to leave a genetic signature. The relatively low level of genetic diversity associated with this species is a concern as it may impair fitness and evolutionary capability in a changing environment. The results of this study provide the foundation for the development of management practices that will assist in the protection of this rare carnivorous plant.




Similar content being viewed by others
References
Alcalá RE, Domínguez CA (2012) Genetic structure of the carnivorous plant Pinguicula moranensis (Lentibulariaceae) on the transvolcanic Mexican Belt. Biochem Genet 50:416–427
Althoff DM, Gitzendanner MA, Segraves KA (2007) The utility of amplified fragment length polymorphisms in phylogenetics: a comparison of homology within and between genomes. Syst Biol 56:477–484
Backs JR, Terry M, Klein M, Ashley MV (2015) Genetic analysis of a rare isolated species: a tough little West Texas oak, Quercus hinckleyi CH Mull. J Torrey Bot Soc 142:302–313
Blanca G, Ruiz-Rejon M, Zamora R (1999) Taxonomic revision of the genus Pinguicula L. in the Iberian Peninsula. Folia Geobot 34:337–361
Casper SJ, Stimper R (2009) Chromosome numbers in Pinguicula (Lentibulariaceae): survey, atlas, and taxonomic conclusions. Plant Syst Evol 277:21–60
Chung MY, Lopez-Pujol J, Chung MG (2013) Population history of the two carnivorous plants Drosera peltata var. nipponica and Drosera rotundifolia (Droseraceae) in Korea. Am J Bot 100:2231–2239
Cieslak T, Polepalli JS, White A, Muller K, Borsch T, Barthlott W, Steiger J, Marchant A, Legendre L (2005) Phylogenetic analysis of Pinguicula (Lentibulariaceae): chloroplast DNA sequences and morphology support several geographically distinct radiations. Am J Bot 92:1723–1736
Conti F, Peruzzi L (2006) Pinguicula (Lentibulariaceae) in central Italy: taxonomic study. Ann Bot Fenn 43:321–337
de Witte LC, Armbruster GFJ, Gielly L, Taberlet P, Stocklin J (2012) AFLP markers reveal high clonal diversity and extreme longevity in four key arctic-alpine species. Mol Ecol 21:1081–1097
Demauro MM (1993) Relationship of breeding system to rarity in the lakeside daisy (Hymenoxys acaulis var. glabra). Conserv Biol 7:542–550
Dray S, Dufour AB (2007) The ade4 package: implementing the duality diagram for ecologists. J Stat Softw 22:1–20
Earl DA, Vonholdt BM (2012) STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Res 4:359–361
Edmands S (2007) Between a rock and a hard place: evaluating the relative risks of inbreeding and outbreeding for conservation and management. Mol Ecol 16:463–475
Ellison AM, Gotelli NJ (2009) Energetics and the evolution of carnivorous plants: Darwin’s ‘most wonderful plants in the world’. J Exp Bot 60:19–42
Escaravage N, Questiau S, Pornon A, Doche B, Taberlet P (1998) Clonal diversity in a Rhododendron ferrugineum L. (Ericaceae) population inferred from AFLP markers. Mol Ecol 7:975–982
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620
Falinska K, Lembicz M, Jarmolowski A, Borkowska L (2010) Patterns of genetic diversity in populations of Filipendula ulmaria (L.) at different stages of succession on a meadow abandoned for 30 years. Pol J Ecol 58:27–40
Fenster CB, Galloway LF (2000) Inbreeding and outbreeding depression in natural populations of Chamaecrista fasciculata (Fabaceae). Conserv Biol 14:1406–1412
Florida Natural Areas Inventory (2010) Guide to the natural communities of Florida, 2010th edn. Florida Natural Areas Inventory, Tallahassee
Fraser DJ, Bernatchez L (2001) Adaptive evolutionary conservation: towards a unified concept for defining conservation units. Mol Ecol 10:2741–2752
Furches MS, Small RL, Furches A (2013) Genetic diversity in three endangered pitcher plant species (Sarracenia; Sarraceniaceae) is lower than widespread congeners. Am J Bot 100:2092–2101
Gitzendanner MA, Soltis PS (2000) Patterns of genetic variation in rare and widespread plant congeners. Am J Bot 87:783–792
Gitzendanner MA, Weekley CW, Germain-Aubrey CC, Soltis DE, Soltis PS (2012) Microsatellite evidence for high clonality and limited genetic diversity in Ziziphus celata (Rhamnaceae), an endangered, self-incompatible shrub endemic to the Lake Wales Ridge, Florida, USA. Conserv Genet 13:223–234
Godfrey RK, Stripling HL (1961) A synopsis of Pinguicula (Lentibulariaceae) in the Southeastern United States. Am Midl Nat 66:395–409
Heslop-Harrison Y (2004) Biological Flora of the British Isles, No. 237: pinguicula L. J Ecol 92:1071–1118
Heslop-Harrison Y, Heslop-Harrison J (1981) The digestive glands of Pinguicula: structure and cytochemistry. Ann Bot 47:293–319
Horner JD, Hodcroft EB, Hale AM, Williams DA (2014) Clonality, genetic variation, and the origin of isolated western populations of the carnivorous plant, Sarracenia alata. J Torrey Bot Soc 141:326–337
Hubisz MJ, Falush D, Stephens M, Pritchard JK (2009) Inferring weak population structure with the assistance of sample group information. Mol Ecol Res 9:1322–1332
Huford KM, Krauss SL, Veneklaas EJ (2012) Inbreeding and outbreeding depression in Stylidium hispidum: implications for mixing seed sources for ecological restoration. Ecol Evol 2:2262–2273
Ilves A, Lanno K, Sammul M, Tali K (2013) Genetic variability, population size and reproduction potential in Ligularia sibirica (L.) populations in Estonia. Conserv Genet 14:661–669
Jennings DE, Rohr JR (2011) A review of the conservation threats to carnivorous plants. Biol Conserv 144:1356–1363
Karron JD (1987) A comparison of levels of genetic polymorphism and self-compatibility in geographically restricted and widespread plant congeners. Evol Ecol 1:47–58
Kephart SR (2004) Inbreeding and reintroduction: progeny success in rare Silene populations of varied density. Conserv Genet 5:49–61
Kesler HC, Trusty JL, Hermann SM, Guyer C (2008) Demographic responses of Pinguicula ionantha to prescribed fire: a regression-design LTRE approach. Oecologia 156:545–557
Kim ES, Zaya DN, Fant JB, Ashley MV (2015) Genetic factors accelerate demographic decline in rare Asclepias species. Conserv Genet 16:359–369
Koopman MM, Carstens BC (2010) Conservation genetic inferences in the carnivorous pitcher plant Sarracenia alata (Sarraceniaceae). Conserv Genet 11:2027–2038
Legendre L (2000) The genus Pinguicula L. (Lentibulariaceae): an overview. Acta Bot Gall 147:77–95
López-Pujol J, Martinell MC, Massó S, Blanché C, Sáez L (2013) The ‘paradigm of extremes’: extremely low genetic diversity in an extremely narrow endemic species, Coristospermum huteri (Umbelliferae). Plant Syst Evol 299:439–446
Lynch M, Milligan BG (1994) Analysis of population genetic structure with RAPD markers. Mol Ecol 3:91–99
Montlavo AM, Ellstrand NC (2001) Nonlocal transplantation and outbreeding depression in the subshrub Lotus scoparius (Fabaceae). Am J Bot 88:258–269
NatureServe (2015) NatureServe Explorer: An online encyclopedia of life [web application]. Version 7.1. NatureServe, Arlington, Virginia. http://explorer.natureserve.org. Accessed: 20 Sept 2016
Nybom H (2004) Comparison of different nuclear DNA markers for estimating intraspecific genetic diversity in plants. Mol Ecol 13:1143–1155
Oksanen J, Guillaume Blanchet F, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H (2014) vegan: Community Ecology Package. R package version 2.2-0. http://CRAN.R-project.org/package=vegan
Paradis E (2010) pegas: an R package for population genetics with an integrated-modular approach. Bioinformatics 26:419–420
Peakall R, Smouse PE (2012) GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics 28:2537–2539
Porras-Hurtado L, Ruiz Y, Santos C, Phillips C, Carracedo Á, Lareu MV. 2013. An overview of STRUCTURE: applications, parameter settings, and supporting software. Frontiers in Genetics, 4, p. 98
Pritchard JK, Stephens M, Rosenberg NA, Donnelly P (2000) Association mapping in structured populations. Am J Hum Genet 67:170–181
R Core Team (2014) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org
Rodondi G, Beretta M, Andreis C (2010) Pollen morphology of alpine butterworts (Pinguicula L., Lentibulariaceae). Rev Palaeobot Palynol 162:1–10
Scariot V, De Keyser E, Handa T, De Riek J (2007) Comparative study of the discriminating capacity and effectiveness of AFLP, STMS and EST markers in assessing genetic relationships among evergreen azaleas. Plant Breeding 126:207–212
Schmidt K, Jensen K (2000) Genetic structure and AFLP variation of remnant populations in the rare plant Pedicularis palustris (Scrophulariaceae) and its relation to population size and reproductive components. Am J Bot 87:678–689
Swofford DL (2002) PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4. Sunderland: Sinauer Associates
Tecic DL, McBride JL, Bowles ML, Nickrent DL (1998) Genetic variability in the federal threatened Mead’s milkweed, Asclepias meadii Torrey (Asclepiadaceae), as determined by allozyme electrophoresis. Ann Mo Bot Gard 85:97–109
United States Fish and Wildlife Service (2009) Pinguicula ionantha (Godfrey’s butterwort). 5-year review: Summary and Evaluation. Panama City, Florida
Vavrek MJ (2011) Fossil: palaeoecological and palaeogeographical analysis tools. Palaeontological Electronica, 14
Vekemans X (2002) AFLP-SURV version 1.0. Laboratoire de Génétique et Ecologie Végétale, Université Libre de Bruxelles, Belgium. http://www.ulb.ac.be/sciences/lagev/aflp-surv.html
Vos P, Hogers R, Bleeker M, Reijans M, Vandelee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M, Zabeau M (1995) AFLP: a new technique for DNA-fingerprinting. Nucleic Acids Res 23:4407–4414
Wunderlin RP, Hansen BF (2008) Pinguicula. In, Atlas of Florida Vascular Plants (http://www.plantatlas.usf.edu/). S. M. Landry and K. N. Campbell (application development), Florida Center for Community Design and Research. Institute for Systematic Botany, University of South Florida, Tampa
Yildirim H, Senol SG, Pirhan AF (2012) Pinguicula habilii (Lentibulariaceae), a new carnivorous species from South-West Anatolia, Turkey. Phytotaxa 64:46–58
Zamora R, Jamilena M, Rejon MR, Blanca G (1996) Two new species of the carnivorous genus Pinguicula, (Lentibulariaceae) from Mediterranean habitats. Plant Syst Evol 200:41–60
Acknowledgments
We would like to thank the following individuals and organizations that made this project possible: Jean Mengelkoch (Illinois Natural History Survey); Michael R. Jenkins and David Morse (Florida Forest Service); Vivian Negron-Ortiz (U.S. Fish and Wildlife Service); Faye Winters (U.S. Bureau of Land Management); Jim Moyers (St. Joe Company); Wendy Jones (Tyndall Air Force Base); Caroline George (U.S. Fish and Wildlife Service); Dylan Shoemaker, Barry Townsend, Sandra Chafin, Lisa Duglecki, Max Prucell, Pat Prucell, Dave Peterson, Joy Peterson, and Matt Green (St. Joseph Bay State Buffer Preserve); Brittany Phillips (Apalachicola National Forest); Andres Botero (New York Botanical Garden). We also thank Mary Ashley, Janet Rizner Backs, John Wilk, Eun Sun Kim, Jason Palagi, Percy Jinga, and Vivian Negron-Ortiz for providing comments that improved the manuscript, and Danielle Ruffatto for assistance with the figures. Lastly, this project was supported by logistical and financial support from Florida Forest Service (FWS Section 6), U.S. Fish and Wildlife Service (Agreement No: FIIAC00685), U.S. Bureau of Land Management, Eastern Illinois University, Augustana College, New York Botanical Garden, Rancho Santa Ana Botanical Garden, University of Illinois, and Illinois Natural History Survey.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zaya, D.N., Molano-Flores, B., Feist, M.A. et al. Assessing genetic diversity for the USA endemic carnivorous plant Pinguicula ionantha R.K. Godfrey (Lentibulariaceae). Conserv Genet 18, 171–180 (2017). https://doi.org/10.1007/s10592-016-0891-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-016-0891-9