Abstract
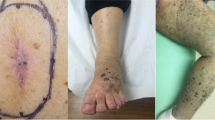
Management of in-transit melanoma encompasses a variety of possible treatment pathways and modalities. Depending on the location of disease, number of lesions, burden of disease and patient preference and characteristics, some treatments may be more beneficial than others. After full body radiographic staging is performed to rule out metastatic disease, curative therapy may be performed through surgical excision, intraarterial regional perfusion and infusion therapies, intralesional injections, systemic therapies or various combinations of any of these. While wide excision is limited in indication to superficial lesions that are few in number, the other listed therapies may be effective in treating unresectable disease. Where intraarterial perfusion based therapies have been shown to successfully treat extremity disease, injectable therapies can be used in lesions of the head and neck. Although systemic therapies for in-transit melanoma have limited specific data to support their primary use for in-transit disease, there are patients who may not be eligible for any of the other options, and current clinical trials are exploring the use of concurrent and sequential use of regional and systemic therapies with early results suggesting a synergistic benefit for oncologic response and outcomes.


Similar content being viewed by others
Data availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
Perone JA, Farrow N, Tyler DS, Beasley GM (2018) Contemporary approaches to in-transit melanoma. J Oncol Pract 14(5):292–300
Wolf IH, Richtig E, Kopera D, Kerl H (2004) Locoregional cutaneous metastases of malignant melanoma and their management. Dermatol Surg 30(2 Pt 2):244–247
Edge SB, Compton CC (2010). The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17(6):1471–1474.
Gershenwald JE, Scolyer RA, Hess KR, et al. (2017) Melanoma staging: evidence-based changes in the american joint committee on cancer eighth edition cancer staging manual. CA Cancer J Clin 67(6):472–492.
Pawlik TM, Ross MI, Thompson JF, Eggermont AMM, Gershenwald JE (2005) The risk of in-transit melanoma metastasis depends on tumor biology and not the surgical approach to regional lymph nodes. J Clin Oncol 23(21):4588–4590
Read RL, Haydu L, Saw RPM et al (2015) In-transit melanoma metastases: incidence, prognosis, and the role of lymphadenectomy. Ann Surg Oncol 22(2):475–481
Stucky C-CH, Gray RJ, Dueck AC et al (2010) Risk factors associated with local and in-transit recurrence of cutaneous melanoma. Am J Surg 200(6):770–775
Thomas JM, Clark MA (2004) Selective lymphadenectomy in sentinel node-positive patients may increase the risk of local/in-transit recurrence in malignant melanoma. Eur J Surg Oncol 30(6):686–691
Pawlik TM, Ross MI, Johnson MM et al (2005) Predictors and natural history of in-transit melanoma after sentinel lymphadenectomy. Ann Surg Oncol 12(8):587–596
Morton DL, Thompson JF, Cochran AJ et al (2014) Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med 370(7):599–609
Speicher PJ, Meriwether CH, Tyler DS (2015) Regional therapies for in-transit disease. Surg Oncol Clin N Am 24(2):309–322
Testori A, Ribero S, Bataille V (2017) Diagnosis and treatment of in-transit melanoma metastases. Eur J Surg Oncol 43(3):544–560
Buzaid AC, Tinoco L, Ross MI, Legha SS, Benjamin RS (1995) Role of computed tomography in the staging of patients with local-regional metastases of melanoma. J Clin Oncol 13(8):2104–2108
Kuvshinoff BW, Kurtz C, Coit DG (1997) Computed tomography in evaluation of patients with stage III melanoma. Ann Surg Oncol 4(3):252–258
Johnson TM, Fader DJ, Chang AE et al (1997) Computed tomography in staging of patients with melanoma metastatic to the regional nodes. Ann Surg Oncol 4(5):396–402
Read RL, Thompson JF (2019) Managing in-transit melanoma metastases in the new era of effective systemic therapies for melanoma. Expert Rev Clin Pharmacol 12(12):1107–1119
Pointer DT Jr, Zager JS (2020) Management of Locoregionally Advanced Melanoma. Surg Clin North Am 100(1):109–125
Augustine CK, Jung SH, Sohn I et al (2010) Gene expression signatures as a guide to treatment strategies for in-transit metastatic melanoma. Mol Cancer Ther 9(4):779–790
Nan Tie E, Henderson MA, Gyorki DE (2019) Management of in-transit melanoma metastases: a review. ANZ J Surg 89(6):647–652
Coit DG, Andtbacka R, Bichakjian CK et al (2009) Melanoma. J Natl Compr Canc Netw 7(3):250–275
Squires MH 3rd, Delman KA (2013) Current treatment of locoregional recurrence of melanoma. Curr Oncol Rep 15(5):465–472
Dong XD, Tyler D, Johnson JL, DeMatos P, Seigler HF (2000) Analysis of prognosis and disease progression after local recurrence of melanoma. Cancer 88(5):1063–1071
Beasley GM, Hu Y, Youngwirth L et al (2017) Sentinel lymph node biopsy for recurrent melanoma: a multicenter study. Ann Surg Oncol 24(9):2728–2733
Creech O Jr, Krementz ET, Ryan RF, Winblad JN (1958) Chemotherapy of cancer: regional perfusion utilizing an extracorporeal circuit. Ann Surg 148(4):616–632
Gabriel E, Skitzki J (2015) The role of regional therapies for in-transit melanoma in the era of improved systemic options. Cancers 7(3):1154–1177
Noorda EM, Vrouenraets BC, Nieweg OE, van Geel BN, Eggermont AMM, Kroon BBR (2004) Isolated limb perfusion for unresectable melanoma of the extremities. Arch Surg 139(11):1237–1242
Moreno-Ramirez D, de la Cruz-Merino L, Ferrandiz L, Villegas-Portero R, Nieto-Garcia A (2010) Isolated limb perfusion for malignant melanoma: systematic review on effectiveness and safety. Oncologist 15(4):416–427
Olofsson R, Mattsson J, Lindnér P (2013) Long-term follow-up of 163 consecutive patients treated with isolated limb perfusion for in-transit metastases of malignant melanoma. Int J Hyperthermia: Off J Eur Soc Hyperther Oncol, North Am Hyperther Group 29(6):551–557
Grunhagen DJ, Verhoef C (2016) Isolated limb perfusion for stage III melanoma: does it still have a role in the present era of effective systemic therapy? Oncology (Williston Park) 30(12):1045–1052
Sanki A, Kam PCA, Thompson JF (2007) Long-term results of hyperthermic, isolated limb perfusion for melanoma: a reflection of tumor biology. Ann Surg 245(4):591–596
Thompson JF, Kam PC, Waugh RC, Harman CR (1998) Isolated limb infusion with cytotoxic agents: a simple alternative to isolated limb perfusion. Semin Surg Oncol 14(3):238–247
Carr MJ, Sun J, Zager JS (2020) Isolated limb infusion: Institutional protocol and implementation. J Surg Oncol
Kroon HM, Coventry BJ, Giles MH et al (2017) Safety and efficacy of isolated limb infusion chemotherapy for advanced locoregional melanoma in elderly patients: an australian multicenter study. Ann Surg Oncol 24(11):3245–3251
Teras J, Kroon HM, Miura JT, et al (2020) International multi-center experience of isolated limb infusion for in-transit melanoma metastases in octogenarian and nonagenarian patients. Ann Surg Oncol (in press)
Teras J, Kroon HM, Zager JS (2020) ASO author reflection: isolated limb infusion for locally advanced melanoma in the extremely old patient is safe and effective. Ann Surg Oncol 27(5):1430–1431
O’Donoghue C, Perez MC, Mullinax JE et al (2017) Isolated limb infusion: a single-center experience with over 200 infusions. Ann Surg Oncol 24(13):3842–3849
Miura JT, Kroon HM, Beasley GM et al (2019) Long-term oncologic outcomes after isolated limb infusion for locoregionally metastatic melanoma: an international multicenter analysis. Ann Surg Oncol 26(8):2486–2494
Carr MJ, Sun J, Kroon HM, et al (2020) Oncologic outcomes after isolated limb infusion for advanced melanoma: an international comparison of the procedure and outcomes between the United States and Australia. Ann Surg Oncol
Carr MJ, Kroon HM, Zager JS (2020) ASO Author reflections: return to isolated limb infusion for in-transit melanoma. Ann Surg Oncol
Beasley GM, Petersen RP, Yoo J et al (2008) Isolated limb infusion for in-transit malignant melanoma of the extremity: a well-tolerated but less effective alternative to hyperthermic isolated limb perfusion. Ann Surg Oncol 15(8):2195–2205
Dossett LA, Ben-Shabat I, Olofsson Bagge R, Zager JS (2016) Clinical response and regional toxicity following isolated limb infusion compared with isolated limb perfusion for in-transit melanoma. Ann Surg Oncol 23(7):2330–2335
Raymond AK, Beasley GM, Broadwater G et al (2011) Current trends in regional therapy for melanoma: lessons learned from 225 regional chemotherapy treatments between 1995 and 2010 at a single institution. J Am Coll Surg 213(2):306–316
Moller MG, Lewis JM, Dessureault S, Zager JS (2008) Toxicities associated with hyperthermic isolated limb perfusion and isolated limb infusion in the treatment of melanoma and sarcoma. Int J Hyperthermia 24(3):275–289
Wieberdink J, Benckhuysen C, Braat RP, van Slooten EA, Olthuis GA (1982) Dosimetry in isolation perfusion of the limbs by assessment of perfused tissue volume and grading of toxic tissue reactions. Eur J Cancer Clin Oncol 18(10):905–910
Hafstrom L, Rudenstam CM, Blomquist E et al (1991) Regional hyperthermic perfusion with melphalan after surgery for recurrent malignant melanoma of the extremities. Swedish Melanoma Study Group. J Clin Oncol 9(12):2091–2094
Santillan AA, Delman KA, Beasley GM et al (2009) Predictive factors of regional toxicity and serum creatine phosphokinase levels after isolated limb infusion for melanoma: a multi-institutional analysis. Ann Surg Oncol 16(9):2570–2578
Chai CY, Deneve JL, Beasley GM et al (2012) A multi-institutional experience of repeat regional chemotherapy for recurrent melanoma of extremities. Ann Surg Oncol 19(5):1637–1643
Belgrano V, Pettersson J, Nilsson JA, Mattsson J, Katsarelias D, Olofsson BR (2019) Response and Toxicity of Repeated Isolated Limb Perfusion (re-ILP) for Patients With In-Transit Metastases of Malignant Melanoma. Ann Surg Oncol 26(4):1055–1062
Ariyan CE, Lefkowitz RA, Panageas K et al (2014) Safety and clinical activity of combining systemic ipilimumab with isolated limb infusion in patients with in-transit melanoma. J Clin Oncol 32(15):9078–9078
Ariyan CE, Brady MS, Siegelbaum RH et al (2018) Robust Antitumor Responses Result from Local Chemotherapy and CTLA-4 Blockade. Cancer Immunol Res 6(2):189
Coley WB (1991) The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. 1893. Clin Orthop Relat Res. 262:3–11
Miura JT, Zager JS (2018) Intralesional therapy as a treatment for locoregionally metastatic melanoma. Expert Rev Anticancer Ther 18(4):399–408
Testori A, Faries MB, Thompson JF et al (2011) Local and intralesional therapy of in-transit melanoma metastases. J Surg Oncol 104(4):391–396
Karakousis CP, Douglass HO Jr, Yeracaris PM, Holyoke ED (1976) BCG immunotherapy in patients with malignant melanoma. Arch Surg 111(6):716–718
Storm FK, Sparks FC, Morton DL (1979) Treatment for melanoma of the lower extremity with intralesional injection of bacille Calmette Guérin and hyperthermic perfusion. Surg Gynecol Obstetr 149(1):17–21
Tan JK, Ho VC (1993) Pooled analysis of the efficacy of bacille Calmette-Guerin (BCG) immunotherapy in malignant melanoma. J Dermatol Surg Oncol 19(11):985–990
Robinson JC (1977) Risks of BCG intralesional therapy: an experience with melanoma. J Surg Oncol 9(6):587–593
Cohen MH, Elin RJ, Cohen BJ (1991) Hypotension and disseminated intravascular coagulation following intralesional bacillus Calmette-Guérin therapy for locally metastatic melanoma. Cancer Immunol Immunother 32(5):315–324
Agarwala SS, Neuberg D, Park Y, Kirkwood JM (2004) Mature results of a phase III randomized trial of bacillus Calmette-Guerin (BCG) versus observation and BCG plus dacarbazine versus BCG in the adjuvant therapy of American Joint Committee on Cancer Stage I-III melanoma (E1673). Cancer 100(8):1692–1698
Liao W, Lin J-X, Leonard WJ (2013) Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity 38(1):13–25
Temple-Oberle CF, Byers BA, Hurdle V, Fyfe A, McKinnon JG (2014) Intra-lesional interleukin-2 therapy for in transit melanoma. J Surg Oncol 109(4):327–331
Tarhini AA, Agarwala SS (2005) Interleukin-2 for the treatment of melanoma. Curr Opin Investig Drugs 6(12):1234–1239
Eklund JW, Kuzel TM (2004) A review of recent findings involving interleukin-2-based cancer therapy. Curr Opin Oncol 16(6):542–546
Byers BA, Temple-Oberle CF, Hurdle V, McKinnon JG (2014) Treatment of in-transit melanoma with intra-lesional interleukin-2: a systematic review. J Surg Oncol 110(6):770–775
Hercus TR, Thomas D, Guthridge MA et al (2009) The granulocyte-macrophage colony-stimulating factor receptor: linking its structure to cell signaling and its role in disease. Blood 114(7):1289–1298
Shi Y, Liu CH, Roberts AI et al (2006) Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: what we do and don’t know. Cell Res 16(2):126–133
Hoeller C, Michielin O, Ascierto PA, Szabo Z, Blank CU (2016) Systematic review of the use of granulocyte-macrophage colony-stimulating factor in patients with advanced melanoma. Cancer Immunol Immunother: CII 65(9):1015–1034
Dranoff G, Jaffee E, Lazenby A et al (1993) Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA 90(8):3539–3543
Si Z, Hersey P, Coates AS (1996) Clinical responses and lymphoid infiltrates in metastatic melanoma following treatment with intralesional GM-CSF. Melanoma Res 6(3):247–255
Nasi ML, Lieberman P, Busam KJ et al (1999) Intradermal injection of granulocyte-macrophage colony-stimulating factor (GM-CSF) in patients with metastatic melanoma recruits dendritic cells. Cytokines Cell Mol Ther 5(3):139–144
Kubin RH, Grodsky GM, Carbone JV (1960) Investigation of Rose Bengal Conjugation. Proc Soc Exp Biol Med 104(4):650–653
Marsh RJ, Fraunfelder FT, McGill JI (1976) Herpetic corneal epithelial disease. Arch Ophthalmol 94(11):1899–1902
Mousavi H, Zhang X, Gillespie S, Wachter E, Hersey P (2006) Rose Bengal induces dual modes of cell death in melanoma cells and has clinical activity against melanoma. Melanoma Res 16
Toomey P, Kodumudi K, Weber A et al (2013) Intralesional injection of rose bengal induces a systemic tumor-specific immune response in murine models of melanoma and breast cancer. PLoS ONE 8(7):e68561
Thompson JF, Hersey P, Wachter E (2008) Chemoablation of metastatic melanoma using intralesional Rose Bengal. Melanoma Res 18(6):405–411
Thompson JF, Agarwala SS, Smithers BM et al (2015) Phase 2 Study of Intralesional PV-10 in Refractory Metastatic Melanoma. Ann Surg Oncol 22(7):2135–2142
Agarwala SS, Ross MI, Zager JS et al (2019) Phase 1b study of PV-10 and anti-PD-1 in advanced cutaneous melanoma. J Clin Oncol 37(15):9559–9559
Agarwala SS, Ross M, Zager JS et al (2020) 1125P A phase Ib study of rose bengal disodium and anti-PD-1 in metastatic cutaneous melanoma: Results in patients naïve to immune checkpoint blockade. Ann Oncol 31:S756
Zager JS, Sarnaik AS, Pilon-Thomas S et al (2020) 1123P A phase Ib study of rose bengal disodium and anti-PD-1 in metastatic cutaneous melanoma: Initial results in patients refractory to checkpoint blockade. Ann Oncol 31:S755–S756
Kohlhapp FJ, Kaufman HL (2016) Molecular pathways: mechanism of action for talimogene laherparepvec, a new oncolytic virus immunotherapy. Clin Cancer Res 22(5):1048–1054
Conry RM, Westbrook B, McKee S, Norwood TG (2018) Talimogene laherparepvec: First in class oncolytic virotherapy. Hum Vaccin Immunother 14(4):839–846
Johnson DB, Puzanov I, Kelley MC (2015) Talimogene laherparepvec (T-VEC) for the treatment of advanced melanoma. Immunotherapy 7(6):611–619
Liu BL, Robinson M, Han ZQ et al (2003) ICP345 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther 10(4):292–303
Hu JC, Coffin RS, Davis CJ et al (2006) A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res 12(22):6737–6747
Senzer NN, Kaufman HL, Amatruda T et al (2009) Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol 27(34):5763–5771
Andtbacka RH, Kaufman HL, Collichio F et al (2015) Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol 33(25):2780–2788
Andtbacka RH, Ross M, Puzanov I et al (2016) Patterns of clinical response with talimogene laherparepvec (T-VEC) in patients with melanoma treated in the OPTiM Phase III clinical trial. Ann Surg Oncol 23(13):4169–4177
Perez MC, Miura JT, Naqvi SMH et al (2018) Talimogene laherparepvec (TVEC) for the treatment of advanced melanoma: a single-institution experience. Ann Surg Oncol 25(13):3960–3965
Louie RJ, Perez MC, Jajja MR et al (2019) Real-world outcomes of talimogene laherparepvec therapy: a multi-institutional experience. J Am Coll Surg 228(4):644–649
Puzanov I, Milhem MM, Minor D et al (2016) Talimogene laherparepvec in combination with ipilimumab in previously untreated, unresectable stage IIIB-IV melanoma. J Clin Oncol 34(22):2619–2626
Chesney J, Puzanov I, Collichio F et al (2018) Randomized, open-label phase II study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. J Clin Oncol 36(17):1658–1667
Ribas A, Dummer R, Puzanov I et al (2017) Oncolytic virotherapy promotes intratumoral t cell infiltration and improves Anti-PD-1 immunotherapy. Cell 170(6):1109-1119.e1110
Long GV, Dummer R, Ribas A et al (2015) A Phase I/III, multicenter, open-label trial of talimogene laherparepvec (T-VEC) in combination with pembrolizumab for the treatment of unresected, stage IIIb-IV melanoma (MASTERKEY-265). J Immunother Cancer 3(Suppl 2):P181–P181
Coit DG, Thompson JA, Algazi A et al (2016) NCCN guidelines insights: melanoma, Version 3.2016. J Natl Compr Canc Netw 14(8):945–958
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12(4):252–264
Weber J (2010) Immune checkpoint proteins: a new therapeutic paradigm for cancer–preclinical background: CTLA-4 and PD-1 blockade. Semin Oncol 37(5):430–439
McClure E, Carr MJ, Zager JS (2020) The MAP kinase signal transduction pathway: promising therapeutic targets used in the treatment of melanoma. Expert Rev Anticancer Ther 20(8):687–701
Long GV, Menzies AM, Nagrial AM et al (2011) Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol 29(10):1239–1246
Eggermont AMM, Chiarion-Sileni V, Grob J-J et al (2016) Prolonged Survival In Stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med 375(19):1845–1855
Schachter J, Ribas A, Long GV et al (2017) Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 390(10105):1853–1862
Weber J, Mandala M, Del Vecchio M et al (2017) Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med 377(19):1824–1835
Wolchok JD, Chiarion-Sileni V, Gonzalez R et al (2017) Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 377(14):1345–1356
Long GV, Hauschild A, Santinami M et al (2017) Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med 377(19):1813–1823
Jiang BS, Beasley GM, Speicher PJ et al (2014) Immunotherapy following regional chemotherapy treatment of advanced extremity melanoma. Ann Surg Oncol 21(8):2525–2531
Weber J, Glutsch V, Geissinger E et al (2020) Neoadjuvant immunotherapy with combined ipilimumab and nivolumab in patients with melanoma with primary or in transit disease. Br J Dermatol 183(3):559–563
Gibney GT, Messina JL, Fedorenko IV, Sondak VK, Smalley KSM (2013) Paradoxical oncogenesis–the long-term effects of BRAF inhibition in melanoma. Nat Rev Clin Oncol 10(7):390–399
Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A (2017) Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 168(4):707–723
Kroon HM, Moncrieff M, Kam PCA, Thompson JF (2008) Outcomes following isolated limb infusion for melanoma. A 14-Year experience. Ann Surg Oncol 15(11):3003
Funding
This paper was not funded.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A.P and M.J.C have no conflicts of interest to disclose. J.S. has received research funding from Amgen. J.S.Z has advisory board relationships with Novartis, Sanofi/Regeneron, Merck; receives research funding from Amgen, Delcath Systems, Philogen, Provectus; consults for Castle Biosciences and Philogen, speaker’s bureau for Castle Biosciences, Pfizer and SunPharma. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Presented at the 8th International Cancer Metastasis Congress in San Francisco, CA, USA from October 25–27, 2019 (http://www.cancermetastasis.org). To be published in an upcoming Special Issue of Clinical and Experimental Metastasis: Novel Frontiers in Cancer Metastasis.
Rights and permissions
About this article
Cite this article
Patel, A., Carr, M.J., Sun, J. et al. In-transit metastatic cutaneous melanoma: current management and future directions. Clin Exp Metastasis 39, 201–211 (2022). https://doi.org/10.1007/s10585-021-10100-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-021-10100-3