Abstract
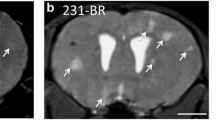
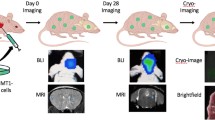
Metastasis is the leading cause of mortality in breast cancer patients, with brain metastases becoming increasingly prevalent. Studying this disease is challenging due to the limited experimental models and methods available. Here, we used iron-based cellular MRI to track the fate of a mammary carcinoma cell line (MDA-MB-231-BR) in vivo to characterize the growth of brain metastases in the nude and severely immune-compromised NOD/SCID/ILIIrg−/− (NSG) mouse. Nude and NSG mice received injections of iron-labeled MDA-MB-231-BR cells. Images were acquired with a 3T MR system and assessed for signal voids and metastases. The percentage of signal voids and the number and volume of metastases were quantified. Ex vivo imaging of the liver, histology, and immunofluorescence labeling was performed. Brain metastases grew more rapidly in NSG mice. At day 21 post cell injection, the average number of brain tumors in NSG mice was approximately four times greater than in nude mice. The persistence of iron-labeled cells, visualized as signal voids by MRI, was also examined. The percentage of voids decreased significantly over time for both nude and NSG mice. Body images revealed that the NSG mice also had metastases in the liver, lungs, and lymph nodes while tumors were only detected in the brains of nude mice. This work demonstrates the advantages of using the highly immune-compromised NSG mouse to study breast cancer metastasis, treatments aimed at inhibiting metastasis and outgrowth of breast cancer metastases in multiple organs, and the role that imaging can play toward credentialing these models that cannot be done with other in vitro or histopathologic methods alone.






Similar content being viewed by others
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- bSSFP:
-
Balanced steady-state free precession
- BW:
-
Bandwidth
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- FA:
-
Flip angle
- FACS:
-
Fluorescence activated cell sorting
- FIESTA:
-
Fast imaging employing steady state acquisition
- GFP:
-
Green fluorescent protein
- H&E:
-
Hematoxylin and eosin
- MPIO:
-
Micron-sized iron oxide particles
- MRI:
-
Magnetic resonance imaging
- NSG:
-
NOD/SCID/ILIIrg−/−
- PBS:
-
Perl’s Prussian Blue
- PDX:
-
Patient-derived xenograft
- RF:
-
Radiofrequency
- TE:
-
Echo time
- TR:
-
Repetition time
- US:
-
Ultrasound
- 231BR:
-
MDA-MB-231BR
References
Patanaphan V, Salazar OM, Risco R (1988) Breast cancer: metastatic patterns and their prognosis. South Med J 81(9):1109–1112
Gavrilovic IT, Posner JB (2005) Brain metastases: epidemiology and pathophysiology. J Neurooncol 75(1):5–14
Nayak L, Lee EQ, Wen PY (2012) Epidemiology of brain metastases. Curr Oncol Rep 14(1):48–54
Tabouret E, Chinot O, Metellus P, Tallet A, Viens P, Goncalves A (2012) Recent trends in epidemiology of brain metastases: an overview. Anticancer Res 32(11):4655–4662
Anders C, Carey LA (2008) Understanding and treating triple-negative breast cancer. Oncology 22(11):1233
Heitz F, Harter P, Lueck H-J, Fissler-Eckhoff A, Lorenz-Salehi F, Scheil-Bertram S et al (2009) Triple-negative and HER2-overexpressing breast cancers exhibit an elevated risk and an earlier occurrence of cerebral metastases. Eur J Cancer 45(16):2792–2798
Loibl S, Gianni L (2017) HER2-positive breast cancer. The Lancet 389(10087):2415–2429
Chambers AF, Groom AC, MacDonald IC (2002) Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2(8):563–572
Goss PE, Chambers AF (2010) Does tumour dormancy offer a therapeutic target? Nat Rev Cancer 10(12):871–877
Fehm T, Mueller V, Marches R, Klein G, Gueckel B, Neubauer H et al (2008) Tumor cell dormancy: implications for the biology and treatment of breast cancer. Apmis 116(7–8):742–753
Brackstone M, Townson JL, Chambers AF (2007) Tumour dormancy in breast cancer: an update. Breast Cancer Res 9(3):208
Lockman PR, Mittapalli RK, Taskar KS, Rudraraju V, Gril B, Bohn KA et al (2010) Heterogeneous blood–tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res 16(23):5664–5678
Burdall SE, Hanby AM, Lansdown MR, Speirs V (2003) Breast cancer cell lines: friend or foe? Breast Cancer Res 5(2):89
Pelleitier M, Montplaisir S (1975) The nude mouse: a model of deficient T-cell function. Methods Achiev Exp Pathol 7:149–166
Yoneda T, Williams PJ, Hiraga T, Niewolna M, Nishimura R (2001) A bone-seeking clone exhibits different biological properties from the MDA-MB-231 parental human breast cancer cells and a brain-seeking clone in vivo and in vitro. J Bone Miner Res 16(8):1486–1495
Kumar S, Bajaj S, Bodla RB (2016) Preclinical screening methods in cancer. Indian J Pharmacol 48(5):481
Gillet J-P, Varma S, Gottesman MM (2013) The clinical relevance of cancer cell lines. J Natl Cancer Inst 105(7):452–458
Siolas D, Hannon GJ (2013) Patient-derived tumor xenografts: transforming clinical samples into mouse models. Cancer Res 73(17):5315–5319
Sulaiman A, Wang L (2017) Bridging the divide: preclinical research discrepancies between triple-negative breast cancer cell lines and patient tumors. Oncotarget 8(68):113269
Weeber F, Ooft SN, Dijkstra KK, Voest EE (2017) Tumor organoids as a pre-clinical cancer model for drug discovery. Cell Chem Biol 24(9):1092–1100
Ruggeri BA, Camp F, Miknyoczki S (2014) Animal models of disease: pre-clinical animal models of cancer and their applications and utility in drug discovery. Biochem Pharmacol 87(1):150–161
Sharpless NE, DePinho RA (2006) The mighty mouse: genetically engineered mouse models in cancer drug development. Nat Rev Drug Discov 5(9):741–754
Dobrolecki LE, Airhart SD, Alferez DG, Aparicio S, Behbod F, Bentires-Alj M et al (2016) Patient-derived xenograft (PDX) models in basic and translational breast cancer research. Cancer Metastasis Rev 35(4):547–573
Whittle JR, Lewis MT, Lindeman GJ, Visvader JE (2015) Patient-derived xenograft models of breast cancer and their predictive power. Breast Cancer Res 17(1):17
Jung J, Seol HS, Chang S (2018) The generation and application of patient-derived xenograft model for cancer research. Cancer Res Treat 50(1):1
Yu J, Qin B, Moyer AM, Sinnwell JP, Thompson KJ, Copland JA et al (2017) Establishing and characterizing patient-derived xenografts using pre-chemotherapy percutaneous biopsy and post-chemotherapy surgical samples from a prospective neoadjuvant breast cancer study. Breast Cancer Res 19(1):130
Contreras-Zárate MJ, Ormond DR, Gillen AE, Hanna C, Day NL, Serkova NJ et al (2017) Development of novel patient-derived xenografts from breast cancer brain metastases. Front Oncol 7:252
Hoffmann J, Fichtner I, Lemm M, Lienau P, Hess-Stumpp H, Rotgeri A et al (2009) Sagopilone crosses the blood–brain barrier in vivo to inhibit brain tumor growth and metastases. Neuro-oncology 11(2):158–166
Naumov GN, MacDonald IC, Weinmeister PM, Kerkvliet N, Nadkarni KV, Wilson SM et al (2002) Persistence of solitary mammary carcinoma cells in a secondary site: a possible contributor to dormancy. Cancer Res 62(7):2162–2168
Shafie SM, Grantham FH (1981) Role of hormones in the growth and regression of human breast cancer cells (MCF-7) transplanted into athymic nude mice. J Natl Cancer Inst 67(1):51–56
Murrell DH, Foster PJ, Chambers AF (2014) Brain metastases from breast cancer: lessons from experimental magnetic resonance imaging studies and clinical implications. J Mol Med 92(1):5–12
Foster-Gareau P, Heyn C, Alejski A, Rutt BK (2003) Imaging single mammalian cells with a 15 T clinical MRI scanner. Magn Reson Med 49(5):968–971
McFadden C, Mallett CL, Foster PJ (2011) Labeling of multiple cell lines using a new iron oxide agent for cell tracking by MRI. Contrast Media Mol Imaging 6(6):514–522
Makela AV, Murrell DH, Parkins KM, Kara J, Gaudet JM, Foster PJ (2016) Cellular imaging with MRI. Top Magn Reson Imaging 25(5):177–186
Heyn C, Ronald JA, Ramadan SS, Snir JA, Barry AM, MacKenzie LT et al (2006) In vivo MRI of cancer cell fate at the single-cell level in a mouse model of breast cancer metastasis to the brain. Magn Reson Med 56(5):1001–1010
Murrell DH, Zarghami N, Jensen MD, Dickson F, Chambers AF, Wong E et al (2017) MRI surveillance of cancer cell fate in a brain metastasis model after early radiotherapy. Magn Reson Med 78(4):1506–1512
Economopoulos V, Chen Y, McFadden C, Foster PJ (2013) MRI detection of nonproliferative tumor cells in lymph node metastases using iron oxide particles in a mouse model of breast cancer. Transl Oncol 6(3):347
Basse P, Hokland P, Heron I, Hokland M (1988) Fate of tumor cells injected into left ventricle of heart in BALB/c mice: role of natural killer cells. JNCI 80(9):657–665
Parkins KM, Hamilton AM, Makela AV, Chen Y, Foster PJ, Ronald JA (2016) A multimodality imaging model to track viable breast cancer cells from single arrest to metastasis in the mouse brain. Sci Rep 6(1):1–9
Murrell DH, Hamilton AM, Mallett CL, van Gorkum R, Chambers AF, Foster PJ (2015) Understanding heterogeneity and permeability of brain metastases in murine models of HER2-positive breast cancer through magnetic resonance imaging: implications for detection and therapy. Transl Oncol 8(3):176–184
Hamilton AM, Parkins KM, Murrell DH, Ronald JA, Foster PJ (2016) Investigating the impact of a primary tumor on metastasis and dormancy using MRI: new insights into the mechanism of concomitant tumor resistance. Tomography 2(2):79
Simpson-Abelson MR, Sonnenberg GF, Takita H, Yokota SJ, Conway TF, Kelleher RJ et al (2008) Long-term engraftment and expansion of tumor-derived memory T cells following the implantation of non-disrupted pieces of human lung tumor into NOD-scid IL2Rγnull mice. J Immunol 180(10):7009–7018
Agliano A, Martin-Padura I, Mancuso P, Marighetti P, Rabascio C, Pruneri G et al (2008) Human acute leukemia cells injected in NOD/LtSz-scid/IL-2Rγ null mice generate a faster and more efficient disease compared to other NOD/scid-related strains. Int J Cancer 123(9):2222–2227
Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ (2008) Efficient tumour formation by single human melanoma cells. Nature 456(7222):593–598
Puchalapalli M, Zeng X, Mu L, Anderson A, Glickman LH, Zhang M et al (2016) NSG mice provide a better spontaneous model of breast cancer metastasis than athymic (nude) mice. PLoS ONE 11(9):e0163521
Henry MN, Chen Y, McFadden CD, Simedrea FC, Foster PJ (2015) In-vivo longitudinal MRI study: an assessment of melanoma brain metastases in a clinically relevant mouse model. Melanoma Res 25(2):127–137
Acknowledgements
The authors would like to thank Ashley V. Makela for assisting with mouse injections for this study and the Canadian Institute for Health Research and the Breast Cancer Society of Canada for their funding.
Funding
This study was supported by the Canadian Institute for Health Research (P.J. Foster) and the Breast Cancer Society of Canada (N.N. Knier).
Author information
Authors and Affiliations
Contributions
PJF designed the experiments. NNK, PJF, and AMH conducted the experiments. NNK analyzed the data. NNK and PJF wrote the main manuscript text. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
Animals were cared for in accordance with the standards of the Canadian Council on Animal. Care, and under an approved protocol of the Western University’s Council on Animal Care (2018-135).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Knier, N.N., Hamilton, A.M. & Foster, P.J. Comparing the fate of brain metastatic breast cancer cells in different immune compromised mice with cellular magnetic resonance imaging. Clin Exp Metastasis 37, 465–475 (2020). https://doi.org/10.1007/s10585-020-10044-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-020-10044-0