Abstract
HOX transcript antisense intergenic RNA (HOTAIR) is a long non-coding RNA (lncRNA) which is increasingly being perceived as a tremendous molecular mediator of brain pathophysiology at multiple levels. Epigenetic regulation of target gene expression carried out by HOTAIR is thorough modulation of chromatin modifiers; histone methyltransferase polycomb repressive complex 2 (PRC2) and histone demethylase lysine-specific demethylase 1 (LSD1). Incidentally, HOTAIR was the first lncRNA shown to elicit sponging of specific microRNA (miRNA or miR) species in a trans-acting manner. It has been extensively studied in various cancers, including gliomas and is regarded as a prominent pro-tumorigenic and pro-oncogenic lncRNA. Indeed, the expression of HOTAIR may serve as glioma grade predictor and prognostic biomarker. The objective of this timely review is not only to outline the multifaceted pathogenic roles of HOTAIR in the development and pathophysiology of gliomas and brain cancers, but also to delineate the research findings implicating it as a critical regulator of overall brain pathophysiology. While the major focus is on neuro-oncology, wherein HOTAIR represents a particularly potent underlying pathogenic player and a suitable therapeutic target, mechanisms underlying the regulatory actions of HOTAIR in neurodegeneration, traumatic, hypoxic and ischemic brain injuries, and neuropsychiatric disorders are also presented.
Graphical Abstract
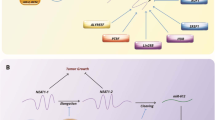
HOTAIR-mediated epigenetic DNA regulation and molecular sponging of target miRNAs. While the 5′ end of HOTAIR regulates the H3K27 trimethylation activity of the catalytic subunit enhancer of Zeste homolog 2 (EZH2) of the polycomb repressive complex 2 (PRC2), its 3′ end modulates the H3K4 demethylation activity of lysine-specific demethylase 1 (LSD1). HOTAIR also binds to and competitively inhibits the functions of target miRNAs, altering the expression of downstream genes.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Long non-coding RNAs (lncRNAs) are RNA species with sizes greater than 200 nucleotides that are not translated into polypeptides. LncRNAs are widely localized at multiple genomic regions; however, they elicit considerable versatility in their biogenesis, cell- and tissue-specific expression patterns, and pathophysiological functions (Bridges et al. 2021). The latest version of Gencode 43 (2022) catalog reports 19,928 lncRNA genes which produce a whopping cumulative total of 58,023 transcripts. The multifaceted cellular functions of lncRNAs are due to their ability to interact with diverse categories of biomolecules, including DNA, mRNA, proteins, and even other non-coding RNAs (e.g., microRNAs or miRNAs) (Herman et al. 2022). One of the prominent mechanisms by which lncRNAs influence cellular functions is by affecting chromatin organization and remodeling, transcription mechanisms, and splicing and export of mRNA species. Interestingly, lncRNAs also regulate the cytoplasmic stability, localization, miRNA-targeting and translation of other RNAs by acting as “molecular sponges” (Borkiewicz et al. 2021). Further, lncRNA species have been reported to induce posttranslational modifications of proteinaceous components (Srinivas et al. 2023). Cells of the central nervous system (CNS) with all their morpho-functional complexities are tremendous targets of lncRNA-mediated alterations in pathophysiological processes (Cuevas-Diaz Duran et al. 2019). Both neurons and glia show abundant expression of various lncRNAs. Indeed, several recent studies have identified prominent roles of different lncRNA species in brain function modulation. LncRNAs have been implicated as intricate and dynamic regulators of multiple aspects of CNS pathophysiology (Cuevas-Diaz Duran et al. 2019), including tumorigenesis (DeSouza et al. 2021; Kim et al. 2021). Because of their multifaceted roles in altering neuronal pathophysiological trajectories, lncRNAs have been envisioned as promising biotargets for diagnosis, prognosis and treatment of multiple neurological disorders (Yang et al. 2021).
HOTAIR (aka HOX transcript antisense intergenic RNA) is generated from the antisense strand of the HOXC gene, which is localized on chromosome 12q13.13 (Price et al. 2021). HOTAIR transcript consists of 6 exons and 2158 nucleotides, and is spliced and polyadenylated (Xin et al. 2021). It was one of the first lncRNAs reported to elicit trans-silencing of genes. Mechanistically, HOTAIR regulates the functions of histone methyltransferase, polycomb repressive complex 2 (PRC2) via its interaction with enhancer of Zeste homolog 2 (EZH2), PRC2’s catalytic subunit (Fig. 1). Specifically, HOTAIR modulates trimethylation activity (at lysine-27 of histone H3; H3K27me3) of PRC2/EZH2, resulting in epigenetic transcriptional repression of the target genes (Rinn et al. 2007). Further, HOTAIR also forms a physical scaffold and aids complex formation of lysine-specific demethylase 1 (LSD1), repressor element 1 silencing transcription factor (REST), and REST corepressor 1 (CoREST1). This results in modulation of the latter’s ability to demethylate lysine-4 of histone H3; H3K4me2 (Tsai et al. 2010). Studies have indicated that while the 5′ domain of HOTAIR binds to and regulates EZH2/PRC2, its 3′ domain is implicated in the regulation of LSD1-REST-CoREST functions. Together, these mechanisms robustly contribute to HOTAIR’s ability to influence epigenetic statuses of numerous target genes (Cai et al. 2014).
The mechanism of epigenetic regulation mediated by HOTAIR lncRNA. 5′ end of HOTAIR is involved in the binding and regulation of H3K27 trimethylation activity of the polycomb repressive complex 2 (PRC2) via the catalytic subunit EZH2. On the other hand, 3′ end of HOTAIR modulates the H3K4 demethylation activity of lysine-specific demethylase 1 (LSD1)—RE1 silencing transcription factor (REST)—REST corepressor 1 (coREST1)
HOTAIR has been proposed to influence diverse pathways in the pathogeneses of multiple types of inflammatory dysfunctions and malignancies [reviewed in Ghafouri-Fard et al. 2021; Price et al. 2021)]. Elevations in the expression and hyperactivation of HOTAIR, and the consequent retargeting of PRC2 and LSD1-REST-coREST1 are thought to be associated with dysregulated epigenetic statuses of underlying genes and aggravation of tumor cell invasiveness and metastases. Conversely, measures directed at repression of HOTAIR expression and activity are related to attenuation of tumorigenesis and cancer progression (Xin et al. 2021). Hence, HOTAIR may be envisioned as a relevant biomarker and therapeutic target in oncological pathogeneses (Lu et al. 2017). Not surprisingly, elevations in HOTAIR levels and the associated detrimental effects on chromatin modification, gene regulation, transcription, RNA processing, and post-transcriptional regulation of downstream genes have been reported to be associated with high tumor grades and severities, as well as poor survival in several cancer types (Hajjari and Salavaty 2015). At the molecular and cellular levels, HOTAIR has been shown to promote tumorigenic pathways via alteration of a variety of signaling cascades (Chen et al. 2021). In fact, HOTAIR represents one of the most prominent and extensively studied lncRNAs which are often found to be hyperactivated in multiple human tumor types, including breast, lung, gastric, colorectal, cervical, and brain cancers.
The objective of the current review is to comprehensively compile the research data implicating HOTAIR lncRNA in a plethora of pathophysiological aspects of brain cancers, particularly gliomas. Relevances of HOTAIR as a prognostic marker and therapeutic target in gliomas and other brain cancers are discussed in detail. Further, the authors summarize the roles of HOTAIR in the pathogeneses of other neuronal disorders, including traumatic brain injury (TBI), psychiatric conditions, and neurodegenerative diseases.
Overview of Tumorigenic Functions of HOTAIR
MiRNA Cross Talk
A wide variety of lncRNA species and their competing endogenous RNA (ceRNA) actions against miRNAs are implicated in the pathophysiology of multiple CNS disorders (Moreno-García et al. 2020; Luo et al. 2021). Incidentally, HOTAIR was one of the first lncRNAs discovered to serve as a “molecular sponge” for inhibiting the activity of multiple miRNA species (Fig. 2). This ability of HOTAIR to act as a ceRNA allows it to abolish availability of target miRNA species for regulating the translation of downstream mRNAs (also see “HOTAIR-Mediated Trans-Silencing of miRNAs in Glioma Pathogeneses” section). For example, HOTAIR competitively binds to miR-20a-5p to block the latter’s interaction with high mobility group A2 (HMGA2) mRNA, thereby increasing the protein levels of HMGA2, and resulting in aggravation of tumorigenicity of breast cancer cells (Zhao et al. 2018). Similarly, HOTAIR has been reported to sequester miR-129-5p, causing pathogenic increases in the levels of Frizzled 7 (FZD7) (Wu et al. 2021).
Epithelial-Mesenchymal Transition (EMT)
EMT is a multistep process associated with forfeit of epithelial characteristics of cells, and acquirement of mesenchymal attributes, such as cellular motility, migration, and invasiveness. The transdifferentiation pathway of EMT is particularly crucial for tumorigenesis, and tumor cell metastasis and migration. Studies indicate that HOTAIR critically influences EMT at multiple levels. Correspondingly, HOTAIR-mediated induction of EMT has been proposed in the pathogeneses of multiple cancers (Amicone et al. 2023). For instance, transforming growth factor beta 1 (TGF-β1)-induced pathogenic overactivation of HOTAIR robustly triggers EMT in colon cancer cells, and repression of HOTAIR correspondingly results in diminution of EMT and metastatic abilities of these cells (Pádua Alves et al. 2013). Similarly, overexpression of HOTAIR in oral squamous cell carcinomas is implicated in increased EMT and invasiveness of tumor cells, while its ablation rescues cellular stemness and tumorigenicity of oral carcinoma cells (Lu et al. 2017). Studies with a dominant negative mutant of HOTAIR in TGF-β-induced EMT have confirmed its ability to bind to and regulate the biological functions of Snail protein (a master regulator of EMT pathway) in the repression of target epithelial genes (Battistelli et al. 2021).
HOTAIR and Gliomas and Other Brain Cancers
Among the primary brain cancers, gliomas represent the most prevalent and heterogeneous cancer types, and are extremely difficult to diagnose, classify, and treat. In recent years, there has been a tremendous surge in the number of studies focused at understanding the pathogenic and protective actions of different lncRNA species (including HOTAIR) in the development of gliomas (Angelopoulou et al. 2020; Cantile et al. 2021).
HOTAIR as a Predictor of Glioma Grade and as a Prognostic Marker
Zhang et al. (2013) were among the first researchers to propose the involvement of HOTAIR in the pathogenesis of gliomas. Further, based upon their observations of its preferential expression in classical and mesenchymal gliomas, rather than the neural and proneural subtypes, they advocated that HOTAIR could serve as a relevant biomarker for differentiating gliomas on the bases of the different gene expression-based molecular subtypes. Moreover, they proposed HOTAIR as a potential marker for tumor grade as its expression was found to be strongly and positively correlated with the tumor grade and severity. Statistical analyses based on correlational Kaplan–Meier survival curve between its expression and survival (hazard ratio; HR 2.933) indicated that HOTAIR lncRNA is a potential prognostic biomarker for predicting survival rates in glioma clinical cases. Mechanistically, employing siRNA-mediated repression of HOTAIR in glioblastoma cell lines (LN229 and U87), the authors found that it regulates cell cycle progression via its inhibitory actions on signaling through transcription factor E2F1, cyclin D1 and E, and cyclin-dependent kinases CDK2 and 4; and via its stimulatory actions on cell cycle proteins, p16 and p21. Lastly, knockdown of HOTAIR under in vivo settings in glioma harboring mice was shown to robustly reduce tumor burden (Zhang et al. 2013). In their follow-up study, the research group identified EZH2/PRC2 as the major molecular target of HOTAIR-mediated pro-oncogenic effects (Zhang et al. 2015). Further, gene ontology (GO) analyses of known targets of HOTAIR confirmed the involvement of physiological cascades of cell proliferation, organization and biogenesis, DNA and RNA metabolism, and cell cycle progression (Zhang et al. 2015). Small nucleolar RNA, C/D box small nucleolar RNA 76 (SNORD76) was identified as another molecular target of HOTAIR signaling. Thus, HOTAIR robustly reduced the expression of SNORD76, and involvement of HOTAIR/SNORD76 signaling in glioblastoma pathogeneses was confirmed both in nude mice with ectopically transplanted U87 cells, and in brain tumor specimens from human subjects (Chen et al. 2015). Further studies indicated that the tumorigenic actions of HOTAIR in gliomas additionally rely on its ability to suppress programmed cell death protein 4 (PDCD4) in a PRC2-dependent manner (Chen et al. 2016). More recently, it was reported that the pro-oncological mediations of HOTAIR likely depend on hyperactivation of β-catenin cascade, possibly in a Nemo-like kinase (NLK)-dependent manner (Zhu et al. 2021).
Analyses of HOTAIR gene expression, methylation status, and copy number in human glioma cases, such as isocitrate dehydrogenase (IDH)-wild type glioblastoma, support the hypothesis of the former’s positive association with glioma grades. Importantly, HOTAIR was confirmed as a prognostic marker for gliomas since patients eliciting its high levels were observed to have significantly decreased survival (Xavier-Magalhães et al. 2018). Further, robust association of the expression of HOTAIR with the levels of homeobox protein HOXA9 was observed in glioma tissues, particularly from clinical subjects of higher-grade gliomas. Subsequent analyses using chromatin immunoprecipitation (ChIP)-qPCR methods indicated direct binding of HOXA9 to the promoter of the HOTAIR gene (Xavier-Magalhães et al. 2018). In concurrence with these results, upon an Arraystar Human lncRNA Microarray-based investigation of the differential expressions of over 33,000 lncRNAs in glioblastoma cases compared to controls, HOTAIR was recognized as a primary lncRNA species with significantly increased expression in glioblastoma (Yan et al. 2015). In consistence, a strong positive association of HOTAIR expression with glioma grades was observed in Chinese clinical cases after adjusting for gender and age (Zhao et al. 2019). Similarly, RNA-seq data from Chinese clinical cases of different grades of gliomas indicated HOTAIR as the top upregulated lncRNA, possibly as a consequence of alterations in its epigenetic DNA methylation pattern (Li et al. 2019b). In contrast, a study reported diminished expression of HOTAIR lncRNA in U118, MG-U87, and MG-LN18 glioblastoma cells; however, it used human parental brain cancer stem cell line as controls (Balci et al. 2016).
An interesting study by Lei et al. (2018) used total RNA sequencing data from 152 glioblastoma subjects from The Cancer Genome Atlas (TCGA) and identified HOTAIR as one of the prominent members in a group of 9 lncRNAs that could potentially be used as a prognostic biomarker set for glioblastoma as well as for predicting patient survival. Further, bioinformatic analyses indicated the possible involvement of several downstream target genes central to a plethora of signaling pathways, such as cell–cell interaction, neurotransmitter physiology, and calcium signaling (Lei et al. 2018). While actual experimental data are needed to confirm these findings and to identify the exact molecular players and pathways linking HOTAIR and glioma development, other in silico studies have also been conducted to propose the multimodal tumor-promoting activities of HOTAIR in gliomas. Using the Chinese Glioma Genome Atlas, Huang et al. (2017) identified HOTAIR as a master regulator of an mRNA network of 18 genes controlling cell cycle progression in the promotion of glioma cell proliferation. These predictions were confirmed experimentally using an in vivo rodent model with glioblastoma xenograft, wherein the authors also observed robust anti-glioma effects of strategies to knockdown HOTAIR expression (Huang et al. 2017).
Computational analyses of lncRNAs as regulators of transcription factor-gene target relationships have resulted in the identification of several lncRNA-transcription factor-gene target axes with a close association with glioblastoma progression, including HOTAIR-MAX interacting protein 1CD 58-protein kinase C epsilon (HOTAIR-MXI 1-CD 58-PRKCE) and HOTAIR-activating transcription factor 5-neural cell adhesion molecule 1 (HOTAIR-ATF 5-NCAM 1) triplets (Li et al. 2016b). These results are in concurrence with a study which used data from TCGA database, and proposed HOTAIR, DLEU1, and LOC00132111 as the lncRNAs with the most significant associations with overall survival of glioma subjects (Lv et al. 2020). Another study based upon data from TCGA database proposed HOTAIR as a prominent lncRNA with robust elevations in the expression levels in glioblastoma subjects (Li et al. 2016a). Interestingly, data extracted from TCGA and Gene Expression Omnibus databases for clinical cases of glioblastomas and low-grade gliomas (LGGs) identified HOTAIR as among the 13 lncRNAs that could potentially distinguish glioblastomas from LGGs (Li et al. 2021).
In conclusion, these data suggest that HOTAIR expression may be a relevant and potent prognosticator of glioma progression, severity, and grade type, either alone or in combination with other biomarkers. Indeed, meta-analysis of systematically reviewed primary data supports this hypothesis (Zhou et al. 2018). In addition, HOTAIR expression as a negative prognostic predictor of poor outcomes in glioma patients raises the possibility of its potential utility as a biotarget for oncological therapies, particularly in gliomas (“HOTAIR-Based Diagnostic and Prognostic Biomarkers from Peripheral Samples” section).
HOTAIR-Mediated Trans-Silencing of miRNAs in Glioma Pathogeneses
As discussed, in addition to the epigenetic mediations, HOTAIR is a prominent component of the ceRNA networks because of its trans-silencing (molecular sponging) ability against multiple miRNAs. This contributes as another interesting facet of HOTAIR-dependent pro-tumorigenic gene expressional effects in gliomas. Below, we delineate, in detail the results from the studies which have focused on molecular sponging-reliant effects of HOTAIR against multiple target miRNA species in glioma pathophysiology (Table 1).
Data from both human glioma tissues and U251 and U87 cells indicate that HOTAIR is responsible for trans-silencing of miR-141. Interestingly, miR-141 may directly bind to and repress the expression of HOTAIR. Such negative regulation of HOTAIR functions mediated by miR-141, possibly dependent on signaling through the spindle and kinetochore-associated protein 2 (SKA2), has been proposed as the underlying cause of its tumor suppression activities. Indeed, alterations in the HOTAIR-miR-141-SKA2 signaling axis by either knockdown of HOTAIR lncRNA or overexpression of miR-141 results in inhibition of growth of ectopic tumor xenografts in mice (Bian et al. 2016). Another molecular sponge of HOTAIR implicated in glioma pathogenesis is miR-326 (Ke et al. 2015). Thus, increases in the levels of HOTAIR in glioma tissues and in vitro tumor cells have been found to be associated with reduced expression of miR-326. Mechanistically, HOTAIR-mediated sponging of miR-326 promotes tumorigenesis by hyperactivating fibroblast growth factor 1 (FGF-1) signaling, resulting in aberrant stimulation of downstream phosphoinositide 3-kinase (PI3K)-Akt and mitogen-activated protein kinases MAPK1/2 cell signaling pathways. Further, inhibition of HOTAIR and the consequent removal of miR-326 silencing were found to protect nude mice challenged with orthotopic tumor transplantation (Ke et al. 2015). Experimental data from A172 glioma cells identified miR-148b-3p as yet another direct mediator of HOTAIR signaling in gliomas. HOTAIR appeared to cause sponging of miR-148b-3p, and silencing the former resulted in diminution of cell survival, proliferative, and metastasis of tumor cells in a miR-148b-3p-dependent manner (Wang et al. 2016). Interestingly, HOTAIR-miR-148b-3p signaling has also been implicated in the dysregulation of blood-tumor barrier in gliomas via the mediation of the upstream stimulatory factor 1 (USF 1) pathway (Sa et al. 2017). Hence, HOTAIR levels were observed to be significantly increased in a glioma model of blood-tumor barrier constructed by co-culture of human cerebral microvascular endothelial hCMEC/D3 cells and U87 cells, in complementation with reduced expression of miR-148b-3p and pathogenic increments in USF 1 function. Proteinaceous components of the tight junctions of microvascular endothelial cells, zonula occludens 1 (ZO 1), occludin, and claudin 5 were also found to be altered. Further, HOTAIR knockdown resulted in normalized miR-148b-3p levels, which in turn promoted rescue of the alterations in USF-1 and tight junction proteins (Sa et al. 2017).
MiR-301a-3p and its downstream target, pro-tumorigenic transcription factor, Fos like 1 (FOSL 1) represent another set of mediators of HOTAIR’s actions in glioma pathogenesis. Thus, transient receptor potential melastatin 7 (TRPM7) channels-induced and HOTAIR upregulation-mediated silencing of miR-301a-3p was found to culminate into significant elevations in the levels of FOSL 1 in glioma tissues (Guo et al. 2022). Similarly, miR-126-5p-glutaminase (GLS) axis has been proposed as a prominent mediator of the pro-oncological actions of HOTAIR (Liu et al. 2018a). HOTAIR as a ceRNA against miR-126-5p promotes GLS expression, as suggested by clinical data from human subjects of astrocytomas, oligodendrogliomas, and glioblastomas; as well as from exploratory studies in glioma cell lines, U87 and U251. Thus, HOTAIR-miR-126-5p-GLS signaling may have significant effects on multiple aspects of glutamine metabolism, aberrant angiogenesis, cellular invasiveness, and temozolomide resistance (see also “HOTAIR as a Mediator of Chemoresistance in Gliomas” section) in glioma pathophysiology (Liu et al. 2018a).
MiR-15b-p53 regulatory loop which is implicated in regulating growth, proliferation, and invasiveness of glioma cells has also been reported to be under the modulatory control of HOTAIR-mediated trans-silencing. It has been observed that induction of miR-15b and p53 promotes apoptosis and inhibits proliferation of U87 cells, while HOTAIR hyperactivity conversely results in increased proliferative and invasive properties of these cells (Sun et al. 2018). MiR-218-phosphodiesterase 7A (PDE7A) signaling axis is another mediator linking hyperactivation of HOTAIR and glioma pathogeneses. Hence, glioma tissue samples and cell lines elicit aberrantly elevated levels of HOTAIR in complementation with significantly repressed levels of miR-218. Direct proof of the involvement of the HOTAIR-miR-218-PDE7A loop in gliomas has been confirmed by both sh-RNA-mediated silencing of HOTAIR and overexpression of miR-218 (Wei et al. 2020). Another recently discovered sponging target of HOTAIR with relevance for gliomas is miR-219, as confirmed by siRNA-mediated silencing of HOTAIR in U87 cells (Li and Guan 2020).
HOTAIR as a Mediator of Chemoresistance in Gliomas
In addition to the pro-tumorigenic effects, several recent studies indicate that HOTAIR signaling plays prominent roles in conferring chemoresistance to tumor cells (Singh et al. 2020). Pharmacological agents have been shown to be rendered severely ineffective because of the hyperactivation of HOTAIR signaling in gliomas. For instance, Yuan et al. (2020) showed that HOTAIR-miR-519a-3p/ribonucleoside diphosphate reductase subunit M1 (RRM1) signaling has been implicated in conferring resistance to human glioblastoma cells, A172 and LN229 against temozolomide. HOTAIR expression was found to be significantly increased in temozolomide resistant glioblastoma cells, and its silencing culminated into regularized vulnerability of the cell and in vivo xenograft tumors to temozolomide. Correspondingly, induction of temozolomide resistance was found to be accorded via HOTAIR-containing exosomes obtained from the temozolomide resistant glioblastoma cells. Further, based upon data from clinical cases of glioblastoma with and without temozolomide resistance, the authors advocated the employment of serum exosomal HOTAIR expression as a diagnostic biomarker for temozolomide sensitivity of glioblastomas (Yuan et al. 2020). This hypothesis seems plausible since extracellular vesicles carrying lncRNAs, among other payload biomolecules, are agents for altering the pathogenic cascades of gliomas and other cancers at multiple levels. Indeed, exposure of serum-derived extracellular vesicles from glioblastoma subjects containing high abundances of HOTAIR was found to robustly facilitate glioma cell proliferation, invasiveness, and temozolomide resistance both in vitro and in vivo (Wang et al. 2022a). Such extracellular vesicular HOTAIR-conferred temozolomide resistance may plausibly be due to attenuation of miR-526b-3p-mediated inhibition of regulator of programmed cell death, EVA 1.
Resistance of gliomas to temozolomide may manifest due to alterations in the HOTAIR-miR125 signaling axis, via hyperactivation of hexokinase 2 (HK2)-mediated glycolytic pathway in tumor cells (Zhang et al. 2020a). Thus, downregulation of HOTAIR was reported to normalization of HK-2 mRNA and protein expressions, and increased temozolomide susceptibility in vitro, in U87 cells, as well as in vivo, in immune-compromised with mice subcutaneous glioma cell xenograft. Lastly, regulatory elements of HOTAIR gene were observed to determine temozolomide resistance in U251 glioma cells, possibly via expressional modulation of distant genes, calcium binding, and coiled-coil domain 1 (CALCOCO1) and zinc finger CCCH-type containing 10 (ZC3H10) (Zhang et al. 2020c).
HOTAIR in Other Brain Cancers
HOTAIR as a pathogenic mediator in pediatric brain tumors has been proposed by Chakravadhanula et al. (2014). They used nanoString platform for transcriptomic analysis in various pediatric brain tumors, and observed significant elevations in HOTAIR levels in atypical teratoid rhabdoid tumors (ATRTs), juvenile pilocytic astrocytomas (JPAs), and medulloblastomas. Ependymomas, on the other hand, were associated with markedly reduced HOTAIR expression (Chakravadhanula et al. 2014). Mechanistic studies in medulloblastoma cells, Daoy and D341 as well as medulloblastoma xenograft mice models to discern the exact molecular mechanisms/players behind the pro-tumorigenic actions of HOTAIR revealed possible involvement of the miR-483-3p-CDK4 axis (Zhao et al. 2020b). Other players implicated in the tumorigenic actions of HOTAIR in medulloblastoma pathogenesis include miR-1 and miR-206, both of which are its sponging targets, and have the capacity to modulate expression of zinc finger transcriptional repressor, Yin Yang 1 (YY1) (Zhang et al. 2020b). Indeed, elevated levels of HOTAIR and YY1 have been observed in tissues from clinical cases of human medulloblastoma, and in Daoy, D283 Med and D341 cell lines. Correspondingly, repression of HOTAIR was observed to alter miR-1/miR-206-YY1 signaling in favor of repression of medulloblastoma cell proliferation, migration and epithelial to mesenchymal transition (EMT), and induction of apoptosis (Zhang et al. 2020b). Lastly, RNA in situ hybridization (RNA-ISH) analyses in ependymoma tissues unveiled significant upregulation of the expression levels of HOTAIR lncRNA; particularly in spinal myxopapillary ependymoma (SMPE), when compared to non-ependymoma tumors of the spinal cord (Zheng et al. 2020). Further studies however are warrantied to establish HOTAIR as a specific diagnostic marker for spinal MPE.
Genetic Variations of HOTAIR and Brain Cancers
HOTAIR SNPs have been proposed to be associated with the development of multiple pathological states, including neuropsychiatric conditions (“Neuropsychiatric Disorders” section) and multiple sclerosis (“Multiple Sclerosis (MS)” section), and various cancer types. One of the pioneering studies focused at evaluation of linkages between HOTAIR polymorphisms and glioma pathogenesis was performed by Xavier-Magalhães et al. (2017). Using Portuguese clinical cases of multiple forms of glioma and controls, they found significant associations of rs920778 CT and rs12826786 CT genotypes with the survival of subjects diagnosed with grade III anaplastic oligodendroglioma. However, no statistically significant differences were observed for distribution of these SNPs per se between the glioma cases and controls. Based upon these findings, the authors propounded the potential utilities of these SNPs as prognostic biomarkers for anaplastic oligodendroglioma (Xavier-Magalhães et al. 2017). Needless to say, future studies are warrantied to evaluate and ascertain the associations of other SNPs of HOTAIR in the development and prognosis of the different types of brain cancers and gliomas.
HOTAIR-Based Diagnostic and Prognostic Biomarkers from Peripheral Samples
As already discussed, elevated expression of HOTAIR is a potent indicator of high grade/severity, poor prognosis, and reduced survival in gliomas. Interestingly, Ren et al. have proposed the use of radiolabeled antisense oligonucleotide probes against HOTAIR in liposomal encapsulations for in vivo real-time imaging of HOTAIR-positive gliomas (Ren et al. 2022).
Liquid biopsy-based peripheral biomarkers represent a minimally invasive and effective source of prognostic and diagnostic information in oncology (Shah et al. 2022), particularly for gliomas and other CNS tumors. Indeed, lncRNAs from peripheral circulations represent a relevant category of prognostic platforms for gliomas (Li et al. 2016a). For instance, assessment of the serum levels of known pro-tumorigenic lncRNAs (including HOTAIR) of 106 clinical cases diagnosed with primary glioblastoma was undertaken by Shen et al. (2018). Regarding HOTAIR, the authors found a strong positive association with recurrence and progression of metastases (adjusted hazard ratio of 1.82), and increased likelihood of mortality (adjusted hazard ratio of 2.04). Further, serum levels of GAS5 lncRNA were found to be linked with diminished tumor recurrence and progression, indicating the possible utilities of circulating HOTAIR and GAS5 in serving as potential reciprocal predictors of disease prognosis and survival in glioblastomas (Shen et al. 2018). Serum-derived exosomes as peripheral prognostic and diagnostic platforms for gliomas, specifically for the evaluation of HOTAIR expression have also been proposed in recent studies (Malissovas et al. 2019). Thus, Tan et al. (2018) measured serum exosomal expression of HOTAIR in glioblastoma multiforme cases, in comparison to control subjects. Area under the ROC curve (AUC) analyses for HOTAIR expression indicated significant differentiation of the cases from the controls with a high specificity and sensitivity.
HOTAIR as a Therapeutic Target
Several recent in vivo and in vitro studies have proposed HOTAIR as a tremendous biotarget for anti-glioma therapies (Angelopoulou et al. 2020). Multiple anti-glioma therapeutic agents/strategies which act by repressing the expression of HOTAIR have been proposed. Fang et al. (2016) synthesized magnetic nanoparticles encapsulating siRNAs against HOTAIR, and demonstrated them to efficiently repress its expression in glioma cells, resulting in robust retardation of tumorigenicity. The anti-tumorigenic actions of these si-HOTAIR-containing magnetic nanoparticles were proposed to be reliant on induction of programmed cell death 4 (PDCD4) in the absence of the HOTAIR-EZH2/LSD1 interactions, and the consequent reductions in the expressions of cell cycle regulators, cyclin D1, Ki67, brain-derived neurotrophic factor (BDNF), and cyclin-dependent kinase 4 (CDK4) (Fang et al. 2016). More recently, hyperactivation of HOTAIR in glioma pathogeneses has been shown to be associated with histone H3K27-methylation-mediated transcriptional repression of peroxisome proliferator-activated receptor alpha (PPARα). This information was used for design of a combinatorial therapeutic strategy of fenofibrate-mediated PPARα activation and siRNA-mediated HOTAIR repression to robustly retard glioma proliferation and invasion (Zhu et al. 2021). Further, a novel strategy for inhibition of pro-oncological activities of HOTAIR has been illustrated by Battistelli et al. (2021). In this study, the authors designed a dominant negative mutant of HOTAIR, rendering it incapable of affecting EZH2-PRC2 complex in the normal manner. Mutant HOTAIR was found to be effective in abolishment of tumor cell motility, invasiveness, and EMT in a Snail protein-dependent manner (Battistelli et al. 2021). Needless to say, more studies are needed to extend the utility of this method in clinical practice. Perhaps, smart glioma-targeting nanoplatforms (Ahmad et al. 2022) might aid in enhancing the specific targeting of this dominant negative HOTAIR mutant to glioma sites in human cases.
HOTAIR is also reported to be a key biotarget of oncolytic virus-mediated glioma therapy. Treatment of glioblastoma U251 cells with HSV-G47∆ oncolytic viral particles results in reduced tumorigenicity in complementation with reductions in the levels of prominent cancer-related lncRNAs, including HOTAIR (Vazifehmand et al. 2022). Evidence also suggests the utility of clustered regularly interspaced short palindromic repeat-based interference (CRISPRi) strategies in identification and subsequent genetic targeting of tumorigenic lncRNAs such as HOTAIR in gliomas (Attenello et al. 2021). Further studies however are warrantied to extend and establish these findings.
Pharmacological studies have also been evaluated/directed to target hyperactivated HOTAIR signaling in gliomas. For instance, cisplatin has been illustrated to inhibit EMT of multiple types of glioblastoma and neuroblastoma cells, via its action on HOTAIR signaling, among other bio-targets (Gonçalves et al. 2020). Bioactive phytochemicals, such as schisandrin B (Sch B) derived from the medicinal plant Schisandra chinensis, have also been proposed to elicit significant anti-glioma repressive actions against HOTAIR sponging of miR-125a-mTOR signaling (Jiang et al. 2017). Molecular docking and simulations combined with experimental analyses have identified AC1Q3QWB (or simply AQB) as a potential inhibitor of HOTAIR-EZH2-PRC2 signaling. In primary patient-derived glioblastoma cells, AQB was found to robustly repress HOTAIR signaling, resulting in beneficial induction of tumor suppressor gene, APC regulator of WNT signaling pathway 2 (APC2), and concomitant suppression of Wnt/β-catenin signaling cascade. In addition, complementation of AQB with 3-deazaneplanocin A (DZNep), an inhibitor of the histone methyltransferase EZH2 resulted in much higher anti-tumorigenic actions, compared to treatment with DZNep alone (Li et al. 2019a). AQB has also been found to induce the expression of tumor suppressor genes, including CWF19-like cell cycle control factor 1 (CWF19L1) which controls cell cycle progression by modulating the stability of cyclin-dependent kinases CDK4/6. Indeed, synergistic actions of AQB and CDK4/6 inhibitor palbociclib were illustrated to induce greater degree of anti-tumorigenic signaling (Shi et al. 2020). In their follow-up study, the research group identified programmed death ligand 1 (PDL-1)-mediated activation of pro-inflammatory nuclear factor kappa B (NF-κB) signaling as a critical HOTAIR-based therapeutic target against immune tolerance of gliomas (Wang et al. 2021b). Further, they reported that AQB could significantly attenuate the upregulation of inflammatory mediators in U87 and TBD glioma cells. AQB-mediated inhibition of HOTAIR was shown to result in increased immune sensitivity and T-cell toxicity of glioma cells, consequently resulting in tumor load reduction and increased survival of immune-compromised mice challenged with orthotopic glioma transplantation. Mechanistic analyses of these AQB-mediated anti-tumorigenic effects suggested that inhibition of HOTAIR signaling may culminate into normalization of the expression levels of ubiquitin regulatory X domain protein 1 (UBXN1) (Wang et al. 2021b). A combinatorial therapeutic regimen of cotreatment with AQB and inhibitor of lysine-specific demethylase 1 or LSD1, GSK-LSD1 has also been propounded as an efficient therapeutic strategy against gliomas (Zhao et al. 2021). Since interaction of HOTAIR with EZH2 is reliant on the former’s functional 5′-domain and its binding to LSD1 requires an intact 3′-domain of HOTAIR, synergistic application of AQB and GSK-LSD1 is expected to result in robust protection against gliomas. Indeed, this therapeutic strategy has been found to efficiently inhibit the expression of cell cycle progression genes as well as activate pro-apoptotic genes both in vitro and in patient-derived xenograft models (Zhao et al. 2021). Another specific small-molecule inhibitor of HOTAIR-EZH2 interaction has been identified in AC1NOD4Q (aka. ADQ) by Ren et al. (2019) using in silico analyses. This inhibitor was found to successfully inhibit H3K27-methylation of NLK, a well-known downstream target of HOTAIR-EZH2 signaling, with consequent inhibition of tumor pathogenesis in a Wnt/β-catenin pathway-dependent manner. Further, experimentation based upon RNA immunoprecipitation (RIP) and electrophoretic mobility shift assay (EMSA) identified the binding site of ADQ to 5′-domian of HOTAIR, which overlaps with the EZH2 binding site (Ren et al. 2019).
Pharmacological strategies to block the activities of bromodomain and extraterminal (BET) proteins are implicated in the therapy against glioblastoma multiforme (Gargano et al. 2023). Evaluation of the molecular mechanisms underlying the anti-oncological effects of BET bromodomain inhibitor I-BET151 in glioblastoma LN18 cells has revealed the significance of abrogation of hyperactivation of HOTAIR lncRNA (Pastori et al. 2015). Indeed, protective effects of I-BET151 were severely compromised by overexpression of HOTAIR. Interestingly, bromodomain containing 4 (BRDA4) protein binds directly to HOTAIR promoter, further supporting the idea that anti-tumorigenic actions of BET protein inhibitors are mediated, at least in part, via altering HOTAIR signaling (Pastori et al. 2015).
HOTAIR in Non-oncological Brain Pathophysiological States
While multiple studies using cell cultures, animal models and human clinical cases have irrefutably established HOTAIR as a prominent pathogenic player in cancers, including gliomas as discussed, a comprehensive understanding of its pathogenic roles in other neuronal disorders has remained largely undiscerned. This is changing as evidenced by the recent surge in studies proposing lncRNAs, including HOTAIR as important mediators of different aspects of non-oncological CNS disorders (Fig. 3), such as neurodegeneration, psychiatric illnesses, and developmental conditions (Li et al. 2020; Wu and Kuo 2020; Canseco-Rodriguez et al. 2022). This section first considers the different non-oncological neuronal pathways influenced by HOTAIR lncRNA. Next, the evidences for involvement of HOTAIR signaling in the pathophysiology of a plethora of non-cancerous neuronal disorders, including neurodegenerative diseases (e.g., Alzheimer’s disease, Parkinson’s disease and multiple sclerosis), traumatic, ischemic and hypoxic brain injuries, and neuropsychiatric conditions, are discussed (Table 2).
HOTAIR as a Modulator of Neuronal Pathways
It is interesting to note that even though HOTAIR is widely expressed in all tissues, brain has one the highest expression levels. Further, HOTAIR expression elicits high brain region specificity and temporal dynamicity (Policarpo et al. 2021). This suggests that HOTAIR presumably has important functions in the regulation of multiple aspects of nervous system development and pathophysiology, not just limited to tumorigenicity. Indeed, recent researches have increasingly uncovered multifaceted regulatory roles of HOTAIR in neurodevelopment, neuronal signaling and plasticity, and neuroinflammation, as delineated below.
Protein phosphorylation as a posttranslational mediator of spatio-temporal regulation of protein presence, stability, interactions, and function has widespread implications for cellular mechanisms. With regard to HOTAIR functions, its interaction with EZH2 has been elicited to be under regulation of the latter’s site-specific phosphorylation status. Thus, binding of HOTAIR to EZH2 is enhanced upon its phosphorylation at threonine-345 (T345) (Kaneko et al. 2010). Since EZH2 is critically implicated as a regulator of neuronal differentiation and regeneration pathways (Yu et al. 2013), it is interesting to hypothesize a prominent posttranslational modification-dependent role of HOTAIR in neuronal maturation, as well as in neuronal regeneration after insults/injuries. This is supported by evidences that HOTAIR-EZH2-miRNA-141 signaling drives expressional changes in BDNF, and regulates the differentiation of dopaminergic neuron-like cells from human amniotic epithelial stem cells (HuAESCs) (Liu et al. 2018b). Along similar lines, Khani‑Habibabadi et al. have recently implicated HOTAIR in the regulation of oligodendrocyte precursor cell differentiation (Khani-Habibabadi et al. 2022).
Evidences suggest that HOTAIR may also influence protein stability, interactions, and functions by targeting their ubiquitin-mediated proteolysis. For instance, HOTAIR has been reported to interact with RNA-binding domains of E3 ubiquitin ligases, DAZ-interacting zinc finger 3 (Dzip3), and Mex-3 RNA-binding family member B (Mex3b), and facilitate ubiquitination and degradation of cellular senescence proteins, ataxin-1 and snurportin-1 (Yoon et al. 2013). Noteworthy, ataxin-1 is a critical modulator of neurodevelopmental cascades and is implicated in various neurological disorders, including spinocerebellar ataxia type 1 (Ju et al. 2014). Also, HOTAIR-Mex3b interactions are thought to alter the function of suppressor of mothers against decapentaplegic family member 4 (SMAD-4) and nucleoside diphosphate linked moiety X-type motif 3 (NUDT-3) (Barrios-Rodiles et al. 2005), further supporting the involvement of HOTAIR in neuronal physiology.
Mediators of inflammatory signaling, cytokines, and chemokines are generated by microglia, astrocytes, and endothelial cells in the CNS and drive neuroinflammatory responses (DiSabato et al. 2016). Association of hyperactivated inflammatory responses with cellular oxidative stress and damage is well established, and pro-inflammatory and prooxidant signaling have been shown to drive pathogenic mechanisms in many diseases (Chatterjee 2016), including CNS disorders. Contributions of lncRNAs, such as HOTAIR to inflammatory and oxidative pathways in CNS pathophysiology are supported by several research studies (Moreno-García et al. 2020; Luo et al. 2021). Hence, HOTAIR may drive microglia activation and regulate the release of inflammatory factors (Cheng et al. 2021). Similarly, pro-inflammatory actions of hyperactivated HOTAIR have been reported to underlie inflammatory injury, oxidative damage, and cellular apoptosis in spinal cord ischemia–reperfusion injury, possibly in a high mobility group box 1 (HMGB-1)- and NF-κB-dependent manner (Wang et al. 2022c).
HOTAIR as a Pathogenic Player in Non-oncological Neuronal Pathologies
Neuropsychiatric Disorders
While the molecular mechanisms have remained undiscerned, clinical studies have indicated probable genetic associations between HOTAIR and pathogeneses of psychiatric disorders. Thus, evaluation of rs1899663, an intronic SNP of HOTAIR revealed its robust association with bipolar disorder type I in the allelic, co-dominant, and dominant models, bipolar disorder type II in allelic and dominant models, and major depressive disorder (MDD) in allelic and dominant genetic models. The SNP was also found to be strongly associated with the risk of developing attention deficit hyperactivity disorder (ADHD) in allelic, co-dominant, and dominant models of inheritance. However, no links between rs1899663 SNP and the development of methamphetamine addiction or schizophrenia were observed in any of the inheritance models assessed (Sayad et al. 2020). Another clinical study in subjects diagnosed with bipolar disorder (and controls) implicated HOTAIR SNPs rs1899663 G/T, rs12826786 C/T, rs4759314 A/G, and rs920778 C/T as risk factors for the development of bipolar disorder under allelic, recessive, dominant, and co-dominant contrasted genetic models (Sargazi et al. 2022). Interestingly, while CT genotype of rs920778 C/T, GT genotype of rs1899663 G/T, and CT genotype of rs12826786 C/T SNPs were proposed to increase the chances of bipolar disorder development, GG genotype of rs4759314 A/G SNP was proposed to attenuate the risk (Sargazi et al. 2022). Autism spectrum disorders (ASDs) are a group of heterogeneous neurodevelopmental behavioral conditions with complex genetic etiologies. In a large-scale genetic study involving 427 ASD clinical cases and 430 normal children, rs12826786 SNP of HOTAIR was reported to be significantly associated with ASD phenotype in allelic (T vs. C; odds ratio, OR 1.29) and recessive (TT vs. TC + CC; OR 1.60) models (Safari et al. 2020). Although these studies provide some evidence for genetic association of HOTAIR lncRNA in the pathogeneses of psychosocial disorders across multiple age groups, future studies must be directed at understanding the underlying mechanism and players linking the two.
Traumatic Brain Injury (TBI)
Comprehensive understanding of lncRNAs as mediators of the pathophysiology of brain injuries, such as stroke and TBI, has been initiated only recently (Zhang et al. 2023). One of the first studies to propose neuroinflammation-stimulating roles of HOTAIR in TBI came from Cheng et al. (2021). They employed Feeney’s free-fall impact method for induction of TBI and elucidated drastic increases in HOTAIR expression levels, concomitantly with elevated microglia-mediated secretion of pro-inflammatory cytokines, IL-1β, IL-6, and TNF-α. Further, these pro-inflammatory effects of HOTAIR were attributed to repressed Nrdp1 (E3 ubiquitin-protein ligase)-mediated ubiquitination and heightened stability of pro-inflammatory protein, myeloid differentiation primary response protein 88 (MyD88). Conversely, silencing of HOTAIR was found to normalize Nrdp1 and MyD88 levels and prevent aberrant microglial activation (Cheng et al. 2021).
Ischemic and Hypoxic Brain Injuries
In rodent hypoxia models of middle cerebral artery occlusion (MCAO), levels of HOTAIR have been shown to be significantly elevated in the ischemic infarction lesion sites. Such increases have been found to be associated with hyperactivation of NADPH oxidase 2 (NOX2), a protein which is thought to drive pathogenic pathways in stroke and ischemic injury to the brain. Further, RNA interference confirmed the roles of HOTAIR–NOX2 interactions in induction of apoptotic pathway in hypoxic cells (Yang and Lu 2016). Molecular sponging of specific miRNAs [e.g., miR-211; (Ma et al. 2020)] mediated by HOTAIR has also been proposed as a key event affecting the pathology of cerebral ischemia–reperfusion injury. For instance, HOTAIR-miR-148p-Kruppel-like factor 6 (KLF6) signaling cascade has been implicated induction of neuronal death following ischemic stroke (Huang et al. 2021). Indeed, hyperactivation of HOTAIR and the consequent sponging of miR-148p/KLF6 is observed in both oxygen-glucose deprived (OGD) cells and MCAO mice, resulting in aberrant activation of inflammatory and apoptotic pathways in a STAT3-dependent manner. Further, knockdown of HOTAIR in MACO mice was linked not only with increased neuronal survival, but also with significant attenuation in the severity of behavioral, sensorimotor, and proprioceptive deficits (Huang et al. 2021). In accordance with these results, human brain microvascular endothelial cells (hBMVECs) when experimentally challenged with OGD/reperfusion (OGD/R) injury showed significant elevations in the abundances of HOTAIR transcripts (Wang et al. 2021a). The consequent hyperactivation of HOTAIR-EZH2/PRC2 signaling induced membrane disintegrity and stimulated pro-apoptotic pathways in the OGD/R endothelial cells. Interestingly, these results of involvement of HOTAIR-EZH2 axis in propagating blood brain barrier (BBB) deficits are supported by clinical data which shows that there are significant increments in HOTAIR levels of plasma samples obtained from neonatal human subjects of hypoxic‑ischemic encephalopathy (HIE) (Wang et al. 2021a). Along similar lines, Ali et al. (2023) have recently evaluated the expressional changes in serum HOTAIR among cerebrovascular stroke (CVS) subjects, with and without chronic hypertension, and controls. Interestingly, they reported diminished plasma levels of HOTAIR transcripts in CVS subjects, compared to the controls. Moreover, stroke subjects with chronic hypertension were found to have reduced HOTAIR levels with respect to those without hypertension (Ali et al. 2023).
Parkinson’s Disease (PD)
PD is the most widely studied neurodegenerative condition when it comes to the evaluation of pathophysiological functions HOTAIR (Zhang et al. 2022), and a plethora of miRNA species have been implicated as targets in HOTAIR-mediated loops of ceRNA networks in PD (Asadi et al. 2023). The pioneering studies linking HOTAIR to PD pathogenesis employed chemical N-methyl-4-phenyl-1,2,3,6- tetrahydropyridine (MPTP)-induced in vivo, and N-methyl-4-phenylpyridinium (MPP+)-induced in vitro SH-SY5Y cellular models. Experimental data from both indicated robust upregulation of HOTAIR, in complementation with hyperactivation of leucine-rich repeat kinase 2 (LRRK2), a well-established pathogenic mediator of PD development. Further, siRNA-mediated repression of HOTAIR elicited significant neuroprotective effects and resulted in the rescue of LRRK2 dysfunction (Liu et al. 2016; Wang et al. 2017). Aberrant HOTAIR signaling in PD has also been linked to altered sponging of miR-126-5p, with the consequent deficits in the activities of RAB3A interacting protein (RAB3IP), a proteinaceous player implicated in synapse development, signaling, and maintenance (Lin et al. 2019). Correspondingly, repression of HOTAIR expression and the beneficial changes in miR-126-5p/RAB3IP signaling have been reported to inhibit the loss of tyrosine hydroxylase (TH)-positive cells and reduce the abundances of α-synuclein-positive cells, in conjugation with significant attenuation of PD-associated decline in motor and behavioral functions (Lin et al. 2019).
Other studies have also reported involvement of additional miRNAs as sponging targets of HOTAIR in the pathophysiology of PD. For example, Lang et al. (2020) showed that elevated HOTAIR expression induces autophagy-related genes (lysosomal-associated membrane protein types I and II; LAMP1 and 2, and microtubule associated protein 1 light chain 3 beta LC3B-I/LC3B-II ratio) in dopaminergic neurons by altering miR-221-3p/neuronal pentraxin II (NPTX2) axis. HOTAIR may also promote NLR family pyrin domain containing 3 (NLRP3)-mediated pyroptosis in neurons as a consequence of its sponging actions targeting miR-326 (Zhang et al. 2021). Further, alterations in the HOTAIR-miR-874-5p-autophagy-related protein 10 (ATG10) signaling axis and aberrant activation of pro-inflammatory, pro-apoptotic, and pro-oxidative signaling have also been observed in MPP+-induced cellular model of PD (Zhao et al. 2020a). More recently, miR-221-3p has been reported as another sponging target of HOTAIR linked to overexpression of α-synuclein and its aberrant aggregation in PD (Sun et al. 2022).
Alzheimer’s Disease (AD)
The first study to link HOTAIR with AD was performed by Lee et al. who evaluated the expressional changes in multiple lncRNA species, and observed significantly dysregulated expression of HOTAIR in 3xTg-AD mice (Lee et al. 2015), a genetic model of familial AD which incorporates both amyloid and tau pathologies. Similar results of elevated HOTAIR levels were obtained in APP/PS1 mice, another genetic model of AD (Lu et al. 2022a). Interestingly, voluntary exercise in these mice was observed to robustly attenuate pro-inflammatory responses and rescue spatial memory deficits, possibly via normalization of hyperactivated HOTAIR and rectification of its sponging actions on the target miR-130a-3p (Lu et al. 2022a). miR-15/107 has also been proposed as a mediator of pathogenic actions of HOTAIR in AD (Spreafico et al. 2018). In this study, cyclin-dependent kinase 5 (Cdk5) was identified as a target of lncRNAs; nuclear-enriched abundant transcript 1 (NEAT1) and HOTAIR. Notably, Cdk5 is a primary focal mediator of tau and amyloid pathologies and is aberrantly hyperactivated in AD. Hence, region-specific alterations in HOTAIR expression in brain tissue samples obtained from clinical subjects of AD may contribute to disease pathology via altering Cdk5 signaling (Spreafico et al. 2018). Further, elevated serum expression of HOTAIR in AD patients has been reported to elicit close associations with the severity of cognitive and memory impairments (Lu et al. 2022b), encouraging the proposition of serum HOTAIR levels as a biomarker for cognitive decline in AD. Interestingly, a 3-month bicycle training paradigm resulted in significant reductions in the serum HOTAIR levels in these clinical subjects, concomitantly with robust attenuation of AD-linked cognitive impairment (Lu et al. 2022b).
It should be noted here that while cognitive decline is a chief phenotypic hallmark of AD, it is not a specific outcome of the pathology. Nevertheless, cognitive enhancement is one of the targets of therapeutic strategies against AD. Although not a model of AD, extended exposure to sevoflurane, which is a volatile anesthetic, is known to induce cognitive dysfunction. Wang et al. (2018) showed that exposure of sevoflurane resulted in diminishment of learning and memory functions and BDNF expression in rats. Further, they illustrated that these sevoflurane-mediated detrimental effects were reliant on HOTAIR-induced dysfunction of REST. In concurrence, siRNA-induced repression of HOTAIR prevented the molecular and behavioral effects of sevoflurane (Wang et al. 2018). Similar results have been observed in isoflurane (another volatile anesthetic)-induced cognitive impairment in rodents. In this study, the authors proposed HOTAIR-mediated sponging of miRNA-129-5p as the major pathogenic event contributing to oxidative dyshomeostasis, hyperactivated neuroinflammatory responses, neuronal death, and behavioral deficits (Wang et al. 2022b).
Multiple Sclerosis (MS)
HOTAIR has also been proposed as a pathogenic player in immune dysfunction and axonal demyelination in MS (Li et al. 2020). In fact, effectiveness of vitamin D3-based therapies in MS patients has been proposed at least in part, to be due to their abilities to normalize the elevated expression of HOTAIR lncRNA (Pahlevan Kakhki et al. 2018). Hence, significant rescue of the upregulated levels of HOTAIR in peripheral blood mononuclear cells (PBMCs) was observed following vitamin D3 supplementation in MS clinical cases. Vitamin D3-mediated modulation of HOTAIR levels was further confirmed in a mouse model of experimental autoimmune encephalomyelitis (Pahlevan Kakhki et al. 2018). Similarly, sulfasalazine, an immunosuppressive pharmacological agent prescribed for MS treatment, was found to significantly decrease HOTAIR expression (Duan et al. 2018). Sulfasalazine treatment in mice subjected to chemically (cuprizone)-induced demyelination resulted in inhibition of pro-inflammatory switch of microglial, and induction of oligodendrocyte differentiation and remyelination pathways. Mechanistically, sulfasalazine was shown to repress HOTAIR expression, thereby affecting its sponging of the miR-136-5p-Akt2-NF-κB cascade (Duan et al. 2018). Clinical data also support the hypothesis of prominent interference of HOTAIR in MS pathology. Thus, it has been reported that rs4759314 SNP of HOTAIR is probably associated with increased risk of developing MS (Taheri et al. 2020). Further, increased expression of HOTAIR has been observed in PBMCs of relapse-remitting MS subjects in the relapse phase, indicating that it could serve as a potential biomarker to differentiate the remit and relapse phases of these subjects (Soltanmoradi et al. 2021).
Diabetic Retinopathy/Neuropathy
Diabetic retinopathy is a serious pathological outcome of hyperglycemia and the consequent damage to retinal cells. Recent studies have provided some evidence for the involvement of HOTAIR in diabetic retinopathy. For instance, appreciable enhancement of HOTAIR expression has been observed in human retinal endothelial cells challenged with high concentrations of extracellular glucose, and in retinal tissues of streptozotocin-induced animal model of diabetic neuropathy. Hyperactivation of HOTAIR was found to be associated with detrimental changes in mitochondrial bioenergetics, redox signaling, and angiogenesis. Correspondingly, RNA interference-mediated repression of HOTAIR prevented these hyperglycemia-associated alterations. Further, samples from serum and vitreous humor derived from clinical subjects of diabetic neuropathy elicited aberrantly enhanced expressions of HOTAIR lncRNA (Biswas et al. 2021). Clinical data have also illustrated the probable linkages of HOTAIR SNPs in diabetic retinopathy. In a case–control study by Chuang et al. (2022) involving subjects with diabetic retinopathy and controls, SNPs rs12427129-CT, rs12427129-CT + TT, and rs1899663-TT were reported to have aberrantly upregulated expression in diabetic retinopathy patients. Moreover, significantly increased presence of SNPs rs12427129-CT + TT and rs1899663-TT were confirmed in clinical subjects of proliferative diabetic retinopathy (Chuang et al. 2022).
Conclusions
Recent studies clarify the multifaceted roles of HOTAIR in neuropathologies. Particularly, its implications as a pro-oncogenic lncRNA are increasingly becoming evident. A plethora of downstream molecular targets and pathways detrimentally affected by HOTAIR during the different stages of glioma have been identified. Nevertheless and in spite of surges in research studies, HOTAIR’s role in other neuronal pathologies has largely remained obscure. This review is a timely and comprehensive attempt to summarize the available research data implicating HOTAIR as an impactful player in the development of multiple neuronal disorders. In doing so, the authors hope that it will serve as a platform for more research studies directed at thorough understandings of the molecular mechanisms underlying HOTAIR-mediated induction of pathological events in the brain. Lastly, we anticipate that further studies are directed to evaluate and establish the utilities of HOTAIR as a potential biotarget for the design of effective multimodal neuroprotective strategies.
Data Availability
Not applicable.
References
Ahmad F, Varghese R, Panda S et al (2022) Smart nanoformulations for brain cancer theranostics: challenges and promises. Cancers 14:5389. https://doi.org/10.3390/cancers14215389
Ali MA, Shaker OG, Khalifa AA et al (2023) LncRNAs NEAT1, HOTAIR, and GAS5 expression in hypertensive and non-hypertensive associated cerebrovascular stroke patients, and its link to clinical characteristics and severity score of the disease. Non-Coding RNA Res 8:96–108. https://doi.org/10.1016/j.ncrna.2022.10.004
Amicone L, Marchetti A, Cicchini C (2023) The lncRNA HOTAIR: a pleiotropic regulator of epithelial cell plasticity. J Exp Clin Cancer Res 42:147. https://doi.org/10.1186/s13046-023-02725-x
Angelopoulou E, Paudel YN, Piperi C (2020) Critical role of HOX transcript antisense intergenic RNA (HOTAIR) in gliomas. J Mol Med 98:1525–1546. https://doi.org/10.1007/s00109-020-01984-x
Asadi MR, Abed S, Kouchakali G et al (2023) Competing endogenous RNA (ceRNA) networks in Parkinson’s disease: a systematic review. Front Cell Neurosci 17:1044634. https://doi.org/10.3389/fncel.2023.1044634
Attenello FJ, Tsung K, Bishara I et al (2021) In vivo CRISPR screening for novel noncoding RNA functional targets in glioblastoma models. J Neurosci Res 99:2029–2045. https://doi.org/10.1002/jnr.24850
Balci T, Yilmaz Susluer S, Kayabasi C et al (2016) Analysis of dysregulated long non-coding RNA expressions in glioblastoma cells. Gene 590:120–122. https://doi.org/10.1016/j.gene.2016.06.024
Barrios-Rodiles M, Brown KR, Ozdamar B et al (2005) High-Throughput mapping of a dynamic signaling network in mammalian cells. Science 307:1621–1625. https://doi.org/10.1126/science.1105776
Battistelli C, Garbo S, Riccioni V et al (2021) Design and functional validation of a mutant variant of the LncRNA HOTAIR to counteract snail function in epithelial-to-mesenchymal transition. Cancer Res 81:103–113. https://doi.org/10.1158/0008-5472.CAN-20-1764
Bian E-B, Ma C-C, He X-J et al (2016) Epigenetic modification of miR-141 regulates SKA2 by an endogenous ‘sponge’ HOTAIR in glioma. Oncotarget 7:30610–30625. https://doi.org/10.18632/oncotarget.8895
Biswas S, Feng B, Chen S et al (2021) The long non-coding RNA HOTAIR is a critical epigenetic mediator of angiogenesis in diabetic retinopathy. Investig Opthalmol Vis Sci 62:20. https://doi.org/10.1167/iovs.62.3.20
Borkiewicz L, Kalafut J, Dudziak K et al (2021) Decoding LncRNAs. Cancers 13:2643. https://doi.org/10.3390/cancers13112643
Bridges MC, Daulagala AC, Kourtidis A (2021) LNCcation: lncRNA localization and function. J Cell Biol 220:e202009045. https://doi.org/10.1083/jcb.202009045
Cai B, Song XQ, Cai JP, Zhang S (2014) HOTAIR: a cancer-related long non-coding RNA. Neoplasma 61:379–391. https://doi.org/10.4149/neo_2014_075
Canseco-Rodriguez A, Masola V, Aliperti V et al (2022) Long non-coding RNAs, extracellular vesicles and inflammation in Alzheimer’s disease. Int J Mol Sci 23:13171. https://doi.org/10.3390/ijms232113171
Cantile M, Di Bonito M, Tracey De Bellis M, Botti G (2021) Functional interaction among lncRNA HOTAIR and MicroRNAs in cancer and other human diseases. Cancers 13:570. https://doi.org/10.3390/cancers13030570
Chakravadhanula M, Ozols VV, Hampton CN et al (2014) Expression of the HOX genes and HOTAIR in atypical teratoid rhabdoid tumors and other pediatric brain tumors. Cancer Genet 207:425–428. https://doi.org/10.1016/j.cancergen.2014.05.014
Chatterjee S (2016) Oxidative stress, inflammation, and disease. In: Oxidative stress and biomaterials. Elsevier, Amsterdam pp 35–58
Chen L, Han L, Wei J et al (2015) SNORD76, a box C/D snoRNA, acts as a tumor suppressor in glioblastoma. Sci Rep 5:8588. https://doi.org/10.1038/srep08588
Chen Y, Bian Y, Zhao S et al (2016) Suppression of PDCD4 mediated by the long non-coding RNA HOTAIR inhibits the proliferation and invasion of glioma cells. Oncol Lett 12:5170–5176. https://doi.org/10.3892/ol.2016.5323
Chen L, Qian X, Wang Z, Zhou X (2021) The HOTAIR lncRNA: a remarkable oncogenic promoter in human cancer metastasis (review). Oncol Lett 21:302. https://doi.org/10.3892/ol.2021.12563
Cheng S, Zhang Y, Chen S, Zhou Y (2021) LncRNA HOTAIR participates in microglia activation and inflammatory factor release by regulating the ubiquitination of MYD88 in traumatic brain injury. J Mol Neurosci 71:169–177. https://doi.org/10.1007/s12031-020-01623-7
Chuang C-C, Wang K, Yang Y-S et al (2022) Association of long noncoding RNA HOTAIR polymorphism and the clinical manifestations of diabetic retinopathy. Int J Environ Res Public Health 19:14592. https://doi.org/10.3390/ijerph192114592
Cuevas-Diaz Duran R, Wei H, Kim DH, Wu JQ (2019) Invited review: long non-coding RNAs: important regulators in the development, function and disorders of the central nervous system. Neuropathol Appl Neurobiol 45:538–556. https://doi.org/10.1111/nan.12541
DeSouza PA, Qu X, Chen H et al (2021) Long, noncoding RNA dysregulation in glioblastoma. Cancers 13:1604. https://doi.org/10.3390/cancers13071604
DiSabato DJ, Quan N, Godbout JP (2016) Neuroinflammation: the devil is in the details. J Neurochem 139:136–153. https://doi.org/10.1111/jnc.13607
Duan C, Liu Y, Li Y et al (2018) Sulfasalazine alters microglia phenotype by competing endogenous RNA effect of miR-136-5p and long non-coding RNA HOTAIR in cuprizone-induced demyelination. Biochem Pharmacol 155:110–123. https://doi.org/10.1016/j.bcp.2018.06.028
Fang K, Liu P, Dong S et al (2016) Magnetofection based on superparamagnetic iron oxide nanoparticle-mediated low lncRNA HOTAIR expression decreases the proliferation and invasion of glioma stem cells. Int J Oncol 49:509–518. https://doi.org/10.3892/ijo.2016.3571
Gargano D, Segatto M, Di Bartolomeo S (2023) Regulation of cell plasticity by bromodomain and extraterminal domain (BET) proteins: a new perspective in glioblastoma therapy. Int J Mol Sci 24:5665. https://doi.org/10.3390/ijms24065665
Ghafouri-Fard S, Dashti S, Farsi M, Taheri M (2021) HOX transcript antisense RNA: an oncogenic lncRNA in diverse malignancies. Exp Mol Pathol 118:104578. https://doi.org/10.1016/j.yexmp.2020.104578
Gonçalves BÔP, Fialho SL, Silvestrini BR et al (2020) Central nervous system (CNS) tumor cell heterogeneity contributes to differential platinum-based response in an in vitro 2D and 3D cell culture approach. Exp Mol Pathol 116:104520. https://doi.org/10.1016/j.yexmp.2020.104520
Guo S, King P, Liang E et al (2022) LncRNA HOTAIR sponges miR-301a-3p to promote glioblastoma proliferation and invasion through upregulating FOSL1. Cell Signal 94:110306. https://doi.org/10.1016/j.cellsig.2022.110306
Hajjari M, Salavaty A (2015) HOTAIR: an oncogenic long non-coding RNA in different cancers. Cancer Biol Med 12:1–9. https://doi.org/10.7497/j.issn.2095-3941.2015.0006
Herman AB, Tsitsipatis D, Gorospe M (2022) Integrated lncRNA function upon genomic and epigenomic regulation. Mol Cell 82:2252–2266. https://doi.org/10.1016/j.molcel.2022.05.027
Huang K, Sun J, Yang C et al (2017) HOTAIR upregulates an 18-gene cell cycle-related mRNA network in glioma. Int J Oncol 50:1271–1278. https://doi.org/10.3892/ijo.2017.3901
Huang Y, Wang Y, Liu X, Ouyang Y (2021) Silencing lncRNA HOTAIR improves the recovery of neurological function in ischemic stroke via the miR-148a-3p/KLF6 axis. Brain Res Bull 176:43–53. https://doi.org/10.1016/j.brainresbull.2021.08.003
Jiang Y, Zhang Q, Bao J et al (2017) Schisandrin B inhibits the proliferation and invasion of glioma cells by regulating the HOTAIR–micoRNA-125a–mTOR pathway. NeuroReport 28:93–100. https://doi.org/10.1097/WNR.0000000000000717
Ju H, Kokubu H, Lim J (2014) Beyond the glutamine expansion: influence of posttranslational modifications of ataxin-1 in the pathogenesis of spinocerebellar ataxia type 1. Mol Neurobiol 50:866–874. https://doi.org/10.1007/s12035-014-8703-z
Kaneko S, Li G, Son J et al (2010) Phosphorylation of the PRC2 component Ezh2 is cell cycle-regulated and up-regulates its binding to ncRNA. Genes Dev 24:2615–2620. https://doi.org/10.1101/gad.1983810
Ke J, Yao Y, Zheng J et al (2015) Knockdown of long non-coding RNA HOTAIR inhibits malignant biological behaviors of human glioma cells via modulation of miR-326. Oncotarget 6:21934–21949. https://doi.org/10.18632/oncotarget.4290
Khani-Habibabadi F, Zare L, Sahraian MA et al (2022) Hotair and Malat1 long noncoding RNAs regulate Bdnf expression and oligodendrocyte precursor cell differentiation. Mol Neurobiol 59:4209–4222. https://doi.org/10.1007/s12035-022-02844-0
Kim S-H, Lim K-H, Yang S, Joo J-Y (2021) Long non-coding RNAs in brain tumors: roles and potential as therapeutic targets. J Hematol Oncol 14:77. https://doi.org/10.1186/s13045-021-01088-0
Lang Y, Li Y, Yu H et al (2020) HOTAIR drives autophagy in midbrain dopaminergic neurons in the substantia nigra compacta in a mouse model of Parkinson’s disease by elevating NPTX2 via miR-221-3p binding. Aging 12:7660–7678. https://doi.org/10.18632/aging.103028
Lee DY, Moon J, Lee S-T et al (2015) Distinct Expression of long non-coding RNAs in an Alzheimer’s disease model. J Alzheimer’s Dis 45:837–849. https://doi.org/10.3233/JAD-142919
Lei B, Yu L, Jung T et al (2018) Prospective series of nine long noncoding RNAs associated with survival of patients with glioblastoma. J Neurol Surg Part A Cent Eur Neurosurg 79:471–478. https://doi.org/10.1055/s-0038-1655549
Li H, Guan C (2020) HOTAIR inhibits the proliferation of glioblastoma cells by targeting miR-219. Cancer Biomark 28:41–47. https://doi.org/10.3233/CBM-190467
Li Q, Jia H, Li H et al (2016a) LncRNA and mRNA expression profiles of glioblastoma multiforme (GBM) reveal the potential roles of lncRNAs in GBM pathogenesis. Tumor Biol 37:14537–14552. https://doi.org/10.1007/s13277-016-5299-0
Li Y, Wang Z, Wang Y et al (2016b) Identification and characterization of lncRNA mediated transcriptional dysregulation dictates lncRNA roles in glioblastoma. Oncotarget 7:45027–45041. https://doi.org/10.18632/oncotarget.7801
Li Y, Ren Y, Wang Y et al (2019a) A compound AC1Q3QWB selectively disrupts HOTAIR-mediated recruitment of PRC2 and enhances cancer therapy of DZNep. Theranostics 9:4608–4623. https://doi.org/10.7150/thno.35188
Li Z, Tan H, Zhao W et al (2019b) Integrative analysis of DNA methylation and gene expression profiles identifies MIR4435-2HG as an oncogenic lncRNA for glioma progression. Gene 715:144012. https://doi.org/10.1016/j.gene.2019.144012
Li Q-W, Lei W, Chen C, Guo W (2020) Recent advances of long noncoding RNAs involved in the development of multiple sclerosis. Chin J Nat Med 18:36–46. https://doi.org/10.1016/S1875-5364(20)30003-0
Li X, Zhang J, Zhang M et al (2021) Construction and comprehensive analysis of a competitive endogenous RNA network to reveal potential biomarkers for the malignant differentiation of glioma. Medicine 100:e27248. https://doi.org/10.1097/MD.0000000000027248
Lin Q, Hou S, Dai Y et al (2019) LncRNA HOTAIR targets miR-126-5p to promote the progression of Parkinson’s disease through RAB3IP. Biol Chem 400:1217–1228. https://doi.org/10.1515/hsz-2018-0431
Liu S, Cui B, Dai Z et al (2016) Long non-coding RNA HOTAIR promotes Parkinson’s disease induced by MPTP through up-regulating the expression of LRRK2. Curr Neurovasc Res 13:115–120. https://doi.org/10.2174/1567202613666160316155228
Liu L, Cui S, Wan T et al (2018a) Long non-coding RNA HOTAIR acts as a competing endogenous RNA to promote glioma progression by sponging miR-126-5p. J Cell Physiol 233:6822–6831. https://doi.org/10.1002/jcp.26432
Liu T, Zhang H, Zheng J et al (2018b) SPION-mediated miR-141 promotes the differentiation of HuAESCs into dopaminergic neuron-like cells via suppressing lncRNA-HOTAIR. J Cell Mol Med 22:2299–2310. https://doi.org/10.1111/jcmm.13512
Lu M-Y, Liao Y-W, Chen P-Y et al (2017) Targeting LncRNA HOTAIR suppresses cancer stemness and metastasis in oral carcinomas stem cells through modulation of EMT. Oncotarget 8:98542–98552. https://doi.org/10.18632/oncotarget.21614
Lu J, Liu L, Chen J et al (2022a) The involvement of lncRNA HOTAIR/miR-130a-3p axis in the regulation of voluntary exercise on cognition and inflammation of Alzheimer’s disease. Am J Alzheimers Dis Other Demen 37:15333175221091424. https://doi.org/10.1177/15333175221091424
Lu J, Liu L, Chen J et al (2022b) LncRNA HOTAIR in exercise-induced neuro-protective function in Alzheimer’s disease. Folia Neuropathol 60:414–420. https://doi.org/10.5114/fn.2022.118961
Luo X, Tu T, Zhong Y et al (2021) ceRNA network analysis shows that lncRNA CRNDE promotes progression of glioblastoma through sponge mir-9–5p. Front Genet 12:617350. https://doi.org/10.3389/fgene.2021.617350
Lv Q-L, Wang L-C, Li D-C et al (2020) Knockdown lncRNA DLEU1 inhibits gliomas progression and promotes temozolomide chemosensitivity by regulating autophagy. Front Pharmacol 11:560543. https://doi.org/10.3389/fphar.2020.560543
Ma NH, Zhang MH, Yang JX et al (2020) Long noncoding RNA HOTAIR sponging miR-211 regulates cerebral ischemia-reperfusion injury. J Biol Regul Homeost Agents 34:2209–2214. https://doi.org/10.23812/20-287-L
Malissovas N, Ninou E, Michail A, Politis PK (2019) Targeting long non-coding RNAs in nervous system cancers: new insights in prognosis, diagnosis and therapy. Curr Med Chem 26:5649–5663. https://doi.org/10.2174/0929867325666180831170227
Moreno-García L, López-Royo T, Calvo AC et al (2020) Competing endogenous RNA networks as biomarkers in neurodegenerative diseases. Int J Mol Sci 21:9582. https://doi.org/10.3390/ijms21249582
Pádua Alves C, Fonseca AS, Muys BR et al (2013) Brief report: the lincRNA Hotair is required for epithelial-to-mesenchymal transition and stemness maintenance of cancer cell lines. Stem Cells 31:2827–2832. https://doi.org/10.1002/stem.1547
Pahlevan Kakhki M, Nikravesh A, Shirvani Farsani Z et al (2018) HOTAIR but not ANRIL long non-coding RNA contributes to the pathogenesis of multiple sclerosis. Immunology 153:479–487. https://doi.org/10.1111/imm.12850
Pastori C, Kapranov P, Penas C et al (2015) The Bromodomain protein BRD4 controls HOTAIR, a long noncoding RNA essential for glioblastoma proliferation. Proc Natl Acad Sci 112:8326–8331. https://doi.org/10.1073/pnas.1424220112
Policarpo R, Sierksma A, De Strooper B, D’Ydewalle C (2021) From junk to function: LncRNAs in CNS health and disease. Front Mol Neurosci 14:714768. https://doi.org/10.3389/fnmol.2021.714768
Price RL, Bhan A, Mandal SS (2021) HOTAIR beyond repression: in protein degradation, inflammation, DNA damage response, and cell signaling. DNA Repair 105:103141. https://doi.org/10.1016/j.dnarep.2021.103141
Ren Y, Wang Y, Zhang J et al (2019) Targeted design and identification of AC1NOD4Q to block activity of HOTAIR by abrogating the scaffold interaction with EZH2. Clin Epigenet 11:29. https://doi.org/10.1186/s13148-019-0624-2
Ren J, Zhang X, Cao J et al (2022) Radiosynthesis of a novel antisense imaging probe targeting LncRNA HOTAIR in malignant glioma. BMC Cancer 22:79. https://doi.org/10.1186/s12885-022-09170-7
Rinn JL, Kertesz M, Wang JK et al (2007) Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell 129:1311–1323. https://doi.org/10.1016/j.cell.2007.05.022
Sa L, Li Y, Zhao L et al (2017) The role of HOTAIR/miR-148b-3p/USF1 on regulating the permeability of BTB. Front Mol Neurosci 10:194. https://doi.org/10.3389/fnmol.2017.00194
Safari M, Noroozi R, Taheri M, Ghafouri-Fard S (2020) The rs12826786 in HOTAIR lncRNA is associated with risk of autism spectrum disorder. J Mol Neurosci 70:175–179. https://doi.org/10.1007/s12031-019-01421-w
Sargazi S, Zahedi Abghari A, Mirinejad S et al (2022) Long noncoding RNA HOTAIR polymorphisms and susceptibility to bipolar disorder: a preliminary case–control study. Nucleosides Nucleotides Nucleic Acids 41:684–701. https://doi.org/10.1080/15257770.2022.2065017
Sayad A, Badrlou E, Ghafouri-Fard S, Taheri M (2020) Association analysis between the rs1899663 polymorphism of HOTAIR and risk of psychiatric conditions in an Iranian population. J Mol Neurosci 70:953–958. https://doi.org/10.1007/s12031-020-01499-7
Shah UJ, Alsulimani A, Ahmad F et al (2022) Bioplatforms in liquid biopsy: advances in the techniques for isolation, characterization and clinical applications. Biotechnol Genet Eng Rev 38:339–383. https://doi.org/10.1080/02648725.2022.2108994
Shen J, Hodges TR, Song R et al (2018) Serum HOTAIR and GAS5 levels as predictors of survival in patients with glioblastoma. Mol Carcinog 57:137–141. https://doi.org/10.1002/mc.22739
Shi J, Lv S, Wu M et al (2020) HOTAIR-EZH2 inhibitor AC1Q3QWB upregulates CWF19L1 and enhances cell cycle inhibition of CDK4/6 inhibitor palbociclib in glioma. Clin Transl Med 10:182–198. https://doi.org/10.1002/ctm2.21
Singh D, Khan MA, Siddique HR (2020) Emerging role of long non-coding RNAs in cancer chemoresistance: unravelling the multifaceted role and prospective therapeutic targeting. Mol Biol Rep 47:5569–5585. https://doi.org/10.1007/s11033-020-05609-x
Soltanmoradi S, Tavakolpour V, Moghadasi AN, Kouhkan F (2021) Expression analysis of NF-κB-associated long noncoding RNAs in peripheral blood mononuclear cells from relapsing-remitting multiple sclerosis patients. J Neuroimmunol 356:577602. https://doi.org/10.1016/j.jneuroim.2021.577602
Spreafico M, Grillo B, Rusconi F et al (2018) Multiple layers of CDK5R1 regulation in Alzheimer’s disease implicate long non-coding RNAs. Int J Mol Sci 19:2022. https://doi.org/10.3390/ijms19072022
Srinivas T, Mathias C, Oliveira-Mateos C, Guil S (2023) Roles of lncRNAs in brain development and pathogenesis: emerging therapeutic opportunities. Mol Ther. https://doi.org/10.1016/j.ymthe.2023.02.008
Sun G, Wang Y, Zhang J et al (2018) MiR-15b/HOTAIR/p53 form a regulatory loop that affects the growth of glioma cells. J Cell Biochem 119:4540–4547. https://doi.org/10.1002/jcb.26591
Sun Q, Zhang Y, Wang S et al (2022) LncRNA HOTAIR promotes α-synuclein aggregation and apoptosis of SH-SY5Y cells by regulating miR-221-3p in Parkinson’s disease. Exp Cell Res 417:113132. https://doi.org/10.1016/j.yexcr.2022.113132
Taheri M, Noroozi R, Sadeghpour S et al (2020) The rs4759314 SNP within Hotair lncRNA is associated with risk of multiple sclerosis. Mult Scler Relat Disord 40:101986. https://doi.org/10.1016/j.msard.2020.101986
Tan SK, Pastori C, Penas C et al (2018) Serum long noncoding RNA HOTAIR as a novel diagnostic and prognostic biomarker in glioblastoma multiforme. Mol Cancer 17:74. https://doi.org/10.1186/s12943-018-0822-0
Tsai M-C, Manor O, Wan Y et al (2010) Long noncoding RNA as modular scaffold of histone modification complexes. Science 329:689–693. https://doi.org/10.1126/science.1192002
Vazifehmand R, Ali DS, Othman Z et al (2022) The evaluation expression of non-coding RNAs in response to HSV-G47∆ oncolytic virus infection in glioblastoma multiforme cancer stem cells. J Neurovirol 28:566–582. https://doi.org/10.1007/s13365-022-01089-w
Wang G, Li Z, Tian N et al (2016) miR-148b-3p inhibits malignant biological behaviors of human glioma cells induced by high HOTAIR expression. Oncol Lett 12:879–886. https://doi.org/10.3892/ol.2016.4743
Wang S, Zhang X, Guo Y et al (2017) The long noncoding RNA HOTAIR promotes Parkinson’s disease by upregulating LRRK2 expression. Oncotarget 8:24449–24456. https://doi.org/10.18632/oncotarget.15511
Wang J, Feng Y, Fu Y, Liu G (2018) Effect of sevoflurane anesthesia on brain is mediated by lncRNA HOTAIR. J Mol Neurosci 64:346–351. https://doi.org/10.1007/s12031-018-1029-y
Wang Y, Mao J, Li X et al (2021a) lncRNA HOTAIR mediates OGD/R-induced cell injury and angiogenesis in a EZH2-dependent manner. Exp Ther Med 23:99. https://doi.org/10.3892/etm.2021.11022
Wang Y, Yi K, Liu X et al (2021b) HOTAIR up-regulation activates NF-κB to induce immunoescape in gliomas. Front Immunol 12:785463. https://doi.org/10.3389/fimmu.2021.785463
Wang X, Yu X, Xu H et al (2022a) Serum-derived extracellular vesicles facilitate temozolomide resistance in glioblastoma through a HOTAIR-dependent mechanism. Cell Death Dis 13:344. https://doi.org/10.1038/s41419-022-04699-8
Wang Y, Zhao S, Li G et al (2022b) Neuroprotective effect of HOTAIR silencing on isoflurane-induced cognitive dysfunction via sponging microRNA-129-5p and inhibiting neuroinflammation. NeuroImmunoModulation 29:369–379. https://doi.org/10.1159/000521014
Wang Z, Long R, Yang Z, Feng C (2022c) lncRNA HOTAIR inhibition by regulating HMGB1/ROS/NF-κB signal pathway promotes the recovery of spinal cord function. Comput Math Methods Med 2022:1–9. https://doi.org/10.1155/2022/4955982
Wei Y, Zhou K, Wang C et al (2020) Adsorption of miR-218 by lncRNA HOTAIR regulates PDE7A and affects glioma cell proliferation, invasion, and apoptosis. Int J Clin Exp Pathol 13:2973–2983
Wu Y-Y, Kuo H-C (2020) Functional roles and networks of non-coding RNAs in the pathogenesis of neurodegenerative diseases. J Biomed Sci 27:49. https://doi.org/10.1186/s12929-020-00636-z
Wu D, Zhu J, Fu Y et al (2021) LncRNA HOTAIR promotes breast cancer progression through regulating the miR-129-5p/FZD7 axis. Cancer Biomark 30:203–212. https://doi.org/10.3233/CBM-190913
Xavier-Magalhães A, Oliveira AI, de Castro JV et al (2017) Effects of the functional HOTAIR rs920778 and rs12826786 genetic variants in glioma susceptibility and patient prognosis. J Neurooncol 132:27–34. https://doi.org/10.1007/s11060-016-2345-0
Xavier-Magalhães A, Gonçalves CS, Fogli A et al (2018) The long non-coding RNA HOTAIR is transcriptionally activated by HOXA9 and is an independent prognostic marker in patients with malignant glioma. Oncotarget 9:15740–15756. https://doi.org/10.18632/oncotarget.24597
Xin X, Li Q, Fang J, Zhao T (2021) LncRNA HOTAIR: a potential prognostic factor and therapeutic target in human cancers. Front Oncol 11:679244. https://doi.org/10.3389/fonc.2021.679244
Yan Y, Zhang L, Jiang Y et al (2015) LncRNA and mRNA interaction study based on transcriptome profiles reveals potential core genes in the pathogenesis of human glioblastoma multiforme. J Cancer Res Clin Oncol 141:827–838. https://doi.org/10.1007/s00432-014-1861-6
Yang L, Lu Z-N (2016) Long non-coding RNA HOTAIR promotes ischemic infarct induced by hypoxia through up-regulating the expression of NOX2. Biochem Biophys Res Commun 479:186–191. https://doi.org/10.1016/j.bbrc.2016.09.023
Yang S, Lim K-H, Kim S-H, Joo J-Y (2021) Molecular landscape of long noncoding RNAs in brain disorders. Mol Psychiatry 26:1060–1074. https://doi.org/10.1038/s41380-020-00947-5
Yoon J-H, Abdelmohsen K, Kim J et al (2013) Scaffold function of long non-coding RNA HOTAIR in protein ubiquitination. Nat Commun 4:2939. https://doi.org/10.1038/ncomms3939
Yu Y, Chou R, Shyu W et al (2013) Smurf2-mediated degradation of EZH2 enhances neuron differentiation and improves functional recovery after ischaemic stroke. EMBO Mol Med 5:531–547. https://doi.org/10.1002/emmm.201201783
Yuan Z, Yang Z, Li W et al (2020) Expression of concern issued: exosome-mediated transfer of long noncoding RNA HOTAIR regulates temozolomide resistance by miR-519a-3p/RRM1 axis in glioblastoma. Cancer Biother Radiopharm. https://doi.org/10.1089/cbr.2019.3499
Zhang J-X, Han L, Bao Z-S et al (2013) HOTAIR, a cell cycle–associated long noncoding RNA and a strong predictor of survival, is preferentially expressed in classical and mesenchymal glioma. Neuro-oncology 15:1595–1603. https://doi.org/10.1093/neuonc/not131
Zhang K, Sun X, Zhou X et al (2015) Long non-coding RNA HOTAIR promotes glioblastoma cell cycle progression in an EZH2 dependent manner. Oncotarget 6:537–546. https://doi.org/10.18632/oncotarget.2681
Zhang J, Chen G, Gao Y, Liang H (2020a) HOTAIR/miR-125 axis-mediated Hexokinase 2 expression promotes chemoresistance in human glioblastoma. J Cell Mol Med 24:5707–5717. https://doi.org/10.1111/jcmm.15233
Zhang J, Li N, Fu J, Zhou W (2020b) Long noncoding RNA HOTAIR promotes medulloblastoma growth, migration and invasion by sponging miR-1/miR-206 and targeting YY1. Biomed Pharmacother 124:109887. https://doi.org/10.1016/j.biopha.2020.109887
Zhang L, He A, Chen B et al (2020c) A HOTAIR regulatory element modulates glioma cell sensitivity to temozolomide through long-range regulation of multiple target genes. Genome Res 30:155–163. https://doi.org/10.1101/gr.251058.119
Zhang Q, Huang X-M, Liao J-X et al (2021) LncRNA HOTAIR promotes neuronal damage through facilitating NLRP3 mediated-pyroptosis activation in Parkinson’s disease via regulation of miR-326/ELAVL1 axis. Cell Mol Neurobiol 41:1773–1786. https://doi.org/10.1007/s10571-020-00946-8
Zhang H, Yao L, Zheng Z et al (2022) The role of non-coding RNAs in the pathogenesis of Parkinson’s disease: recent advancement. Pharmaceuticals 15:811. https://doi.org/10.3390/ph15070811
Zhang L, Bai W, Sun L et al (2023) Targeting non-coding RNA for CNS injuries: regulation of blood-brain barrier functions. Neurochem Res 48:1997–2016. https://doi.org/10.1007/s11064-023-03892-1
Zhao W, Geng D, Li S et al (2018) LncRNA HOTAIR influences cell growth, migration, invasion, and apoptosis via the miR-20a-5p/ HMGA2 axis in breast cancer. Cancer Med 7:842–855. https://doi.org/10.1002/cam4.1353
Zhao W-H, Yuan H-Y, Ren X-Y et al (2019) Association between expression of HOTAIR and invasiveness of gliomas, and its predictive value. Adv Clin Exp Med 28:1179–1183. https://doi.org/10.17219/acem/99527
Zhao J, Li H, Chang N (2020a) LncRNA HOTAIR promotes MPP+-induced neuronal injury in Parkinson’s disease by regulating the miR-874–5p/ATG10 axis. EXCLI J 19:1141–1153. https://doi.org/10.17179/excli2020-2286
Zhao L, Chen T, Tang X et al (2020b) Medulloblastoma malignant biological behaviors are associated with HOTAIR/miR-483–3p/CDK4 axis. Ann Transl Med 8:886. https://doi.org/10.21037/atm-20-5006
Zhao J, Jin W, Yi K et al (2021) Combination LSD1 and HOTAIR-EZH2 inhibition disrupts cell cycle processes and induces apoptosis in glioblastoma cells. Pharmacol Res 171:105764. https://doi.org/10.1016/j.phrs.2021.105764
Zheng H, Baranova K, Song J et al (2020) Overexpression of long noncoding RNA HOTAIR is a unique epigenetic characteristic of myxopapillary ependymoma. J Neuropathol Exp Neurol 79:1193–1202. https://doi.org/10.1093/jnen/nlaa103
Zhou Q, Liu J, Quan J et al (2018) lncRNAs as potential molecular biomarkers for the clinicopathology and prognosis of glioma: a systematic review and meta-analysis. Gene 668:77–86. https://doi.org/10.1016/j.gene.2018.05.054
Zhu W, Zhao H, Xu F et al (2021) The lipid-lowering drug fenofibrate combined with si-HOTAIR can effectively inhibit the proliferation of gliomas. BMC Cancer 21:664. https://doi.org/10.1186/s12885-021-08417-z
Acknowledgements
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the Project Number ISP23-101.
Funding
This study was funded by Deputyship for Research & Innovation, Ministry of Education, Saudi Arabia—Project Number: ISP23-101.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection and analysis were performed by FA and SR. The first draft of the manuscript was written by FA and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical Approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ahmad, F., Sudesh, R., Ahmed, A.T. et al. Roles of HOTAIR Long Non-coding RNA in Gliomas and Other CNS Disorders. Cell Mol Neurobiol 44, 23 (2024). https://doi.org/10.1007/s10571-024-01455-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10571-024-01455-8