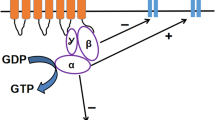
Abstract
The use of morphine as a first-line agent for moderate-to-severe pain is limited by the development of analgesic tolerance. Initially opioid receptor desensitization in response to repeated stimulation, thought to underpin the establishment of tolerance, was linked to a compensatory increase in adenylate cyclase responsiveness. The subsequent demonstration of cross-talk between N-methyl-d-aspartate (NMDA) glutamate receptors and opioid receptors led to the recognition of a role for nitric oxide (NO), wherein blockade of NO synthesis could prevent tolerance developing. Investigations of the link between NO levels and opioid receptor desensitization implicated a number of events including kinase recruitment and peroxynitrite-mediated protein regulation. Recent experimental advances and the identification of new cellular constituents have expanded the potential signaling candidates to include unexpected, intermediary compounds not previously linked to this process such as zinc, histidine triad nucleotide-binding protein 1 (HINT1), micro-ribonucleic acid (mi-RNA) and regulator of G protein signaling Z (RGSZ). A further complication is a lack of consistency in the protocols used to create tolerance, with some using acute methods measured in minutes to hours and others using days. There is also an emphasis on the cellular changes that are extant only after tolerance has been established. Although a review of the literature demonstrates a lack of spatio-temporal detail, there still appears to be a pivotal role for nitric oxide, as well as both intracellular and intercellular cross-talk. The use of more consistent approaches to verify these underlying mechanism(s) could provide an avenue for targeted drug development to rescue opioid efficacy.



Similar content being viewed by others
References
Abbadie C, Pan Y-X, Drake CT, Pasternak GW (2000a) Comparative immunohistochemical distributions of carboxy terminus epitopes from the mu-opioid receptor splice variants MOR-1D, MOR-1 and MOR-1C in the mouse and rat CNS. Neuroscience 100(1):141–153
Abbadie C, Pan Y-X, Pasternak GW (2000b) Differential distribution in rat brain of mu opioid receptor carboxy terminal splice variants MOR-1C-like and MOR-1-like immunoreactivity: evidence for region-specific processing. J Comp Neurol 419(2):244–256
Ahmadi S, Miraki F, Rostamzadeh J (2016) Association of morphine-induced analgesic tolerance with changes in gene expression of GluN1 and MOR1 in rat spinal cord and midbrain. Iran J Basic Med Sci 19:924–931
Ajit SK, Ramineni S, Edris W, Hunt RA, Hum W-T, Hepler JR, Young KH (2007) RGSZ1 interacts with protein kinase C interacting protein PKCI-1 and modulates mu opioid receptor signaling. Cell Signal 19:723–730
Alvarez VA, Arttamangkul S, Dang V, Salem A, Whistler JL, von Zastrow M, Grandy DK, Williams JT (2002) μ-Opioid receptors: ligand-dependent activation of potassium conductance, desensitization, and internalization. J Neurosci 22(13):5769–5776
Aquilante CL, Letrent SL, Pollack GM, Brouwer KLR (2000) Increased brain P-glycoprotein in morphine tolerant rats. Life Sci 66(4):47-P51
Arthur J, Hui D (2018) Safe opioid use: management of opioid-related adverse effects and aberrant behaviors. Hematol Oncol Clin N Am 32(3):387–403
Askitopoulou H, Ramoutsaki IA, Konsolaki E (2002) Archaelogical evidence on the use of opium in the Minoan world. Int Congr 1242:23–29
Babey AM, Kolesnikov Y, Cheng J, Inturrisi CE, Trifilletti RR, Pasternak GW (1994) Nitric oxide and opioid tolerance. Neuropharmacology 33(11):1463–1470
Bagley EE, Chieng BCH, Christie MJ, Connor M (2005) Opioid tolerance in periaqueductal gray neurons isolated from mice chronically treated with morphine. Br J Pharmacol 146:68–76
Bailey CP, Couch D, Johnson E, Griffiths K, Kelly E, Henderson G (2003) μ-Opioid receptor desensitization in mature rat neurons: lack of interaction between DAMGO and morphine. J Neurosci 23(33):10515–10520
Bailey CP, Kelly E, Henderson G (2004) Protein kinase C activation enhances morphine-induced rapid desensitization of μ-opioid receptors in mature rat locus ceruleus neurons. Mol Pharmacol 66(6):1592–1598
Bailey CP, Llorente J, Gabra BH, Smith FL, Dewey WL, Kelly E, Henderson GH (2009a) Role of protein kinase C and μ-opioid receptor (MOPr) desensitization in tolerance to morphine in rat locus coeruleus neurons. Eur J Neurosci 29(2):307–318
Bailey CP, Oldfield S, Llorente J, Caunt CJ, Teschemacher AG, Roberts L, McArdle CA, Smith FL, Dewey WL, Kelly E, Henderson G (2009b) Involvement of PKCa and G-protein-coupled receptor kinase 2 in agonist-selective desensitization of μ-opioid receptors in mature brain neurons. Br J Pharmacol 158:157–164
Bajic D, Berde CB, Commons KG (2012) Periaqueductal gray neuroplasticity following chronic morphine varies with age: role of oxidative stress. Neuroscience 226:165–177
Bajo M, Crawford EF, Roberto M, Madamba SG, Siggins GR (2006) Chronic morphine treatment alters expression of N-methyl-d-aspartate receptor subunits in the extended amygdala. J Neurosci Res 83(4):532–537
Bandyopadhyay S (2019) An 8,000-year history of use and abuse of opium and opioids: how that matters for a successful control of the epidemic? Neurology 92(15 Suppl):P4.9-055
Beckerman MA, Glass MJ (2012) The NMDA-NR1 receptor subunit and the mu-opioid receptor are expressed in somatodendritic compartments of the central nucleus of the amygdala neurons projecting to the bed nucleus of the stria terminalis. Exp Neurol 234(1):112–126
Beirith A, Santos ARS, Calixto JB (2002) Mechanisms underlying the nociception and paw oedema caused by injection of glutamate into the mouse paw. Brain Res 924(2):219–228
Blanchet C, Sollini M, Lüscher C (2003) Two distinct forms of desensitization of G-protein coupled inwardly rectifying potassium currents evoked by alkaloid and peptide mu-opioid receptor agonists. Mol Cell Neurosci 24(2):517–523
Blendy JA, Maldonado R (1998) Genetic analysis of drug addiction: the role of cAMP response element binding protein. J Mol Med 76(2):104–110
Bohn LM, Galnetdinov RR, Lin F-T, Lefkowitz RJ, Caron MJ (2000) μ-Opioid receptor desensitization by β-arrestin-2 determines morphine tolerance but not dependence. Nature 408(6813):720–723
Bohn LM, Lefkowitz RJ, Caron MG (2002) Differential mechanisms of morphine antinociceptive tolerance revealed in β-arrestin-2 knock-out mice. J Neurosci 22(23):10494–10500
Borglund SL, Connor M, Osborne PB, Furness JB, Christie MJ (2003) Opioid agonists have different efficacy profiles for G protein activation, rapid desensitization, and endocytosis of mu-opioid receptors. J Biol Chem 278(2):18776–18784
Bosse KE, Oginsky MF, Susick LL, Ramalingam S, Ferrario CR, Conti AC (2017) Adenylyl cyclase 1 is required for ethanol-induced locomotor sensitization and associated increases in NMDA receptor phosphorylation and function in the dorsal medial striatum. J Pharmacol Exp Ther 363(2):148–155
Bossy-Wetzel E, Talantova MV, Lee WD, Schölzke MN, Harrop A, Mathews E, Götz T, Han J, Ellisman MH, Perkins GA, Lipton SA (2004) Crosstalk between nitric oxide and zinc pathways to neuronal cell death involving mitochondrial dysfunction and p38-activated K+ channels. Neuron 41:351–365
Brook K, Bennett J, Desai SP (2017) The chemical history of morphine: An 8000-year journey, from resin to de-novo synthesis. J Anesth Hist 3:50–55
Brownstein MJ (1993) A brief history of opiates, opioid peptides, and opioid receptors. Proc Nat Acad Sci USA 90:5391–53093
Célérier E, González JR, Maldonado R, Cabañero D, Puig MM (2006) Opioid-induced hyperalgesia in a murine model of postoperative pain role of nitric oxide generated from the inducible nitric oxide synthase. Anesthesiology 104(3):546–555
Chakrabarti S, Gintzler AR (2003) Phosphorylation of Gβ is augmented by chronic morphine and enhances Gβγ stimulation of adenylyl cyclase activity. Mol Brain Res 119(2):144–151
Chakrabarti S, Gintzler AR (2007) Phosphorylation of Gαs influences its association with the μ-opioid receptor and is modulated by long-term morphine exposure. Mol Pharmacol 72(3):753–760
Chao CC, Hu S, Molitor TW, Shaskan EG, Petersen PK (1992) Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J Immunol 149(8):2736–2741
Chao CC, Hu S, Shark KB, Sheng WS, Gekker G, Peterson PK (1997) Activation of mu opioid receptors inhibits microglial cell chemotaxis. J Pharmacol Exp Ther 281(2):998–1004
Chapman CR, Bradshaw DH (2013) Only modest long-term opioid dose escalation occurs over time in chronic non-malignant pain management. J Pain Palliat Care Pharmacother 27(4):370–377
Chen Y, Mestek A, Liu J, Hurley JA, Yu L (1993) Molecular cloning and functional expression of a mu-opioid receptor from rat brain. Mol Pharmacol 44(1):8–12
Chen Z, Muscoli C, Doyle T, Bryant L, Cuzzocrea S, Mollace V, Mastroianni R, Masini E, Salvemini D (2010) NMDA-receptor activation and nitroxidative regulation of the glutamatergic pathway during nociceptive processing. Pain 149(1):100–106
Chen Y-J, Oldfield S, Butcher AJ, Tobin AB, Saxena K, Gurevich VV, Benovic JL, Henderson G, Kelly E (2013) Identification of phosphorylation sites in the COOH-terminal tail of the μ-opioid receptor. J Neurochem 124(2):189–199
Christie MJ, Williams JT, North RA (1987) Cellular mechanisms of opioid tolerance: studies in single brain neurons. Mol Pharmacol 21(5):633–638
Cohen M, Keats AS, Krivoy W, Ungar G (1965) Effects of actinomycin D on morphine tolerance. Proc Soc Exp Biol Med 119:381–384
Collier HOJ (1980) Cellular site of opiate dependence. Nature 283(5748):625–629
Collier HOJ, Roy AC (1974) Morphine-like drugs inhibit the stimulation of E prostaglandins of cyclic AMP formation in rat brain homogenate. Nature 248(5443):24–27
Comb M, Birnberg NC, Seasholtz A, Herbert E, Goodman HM (1986) A cyclic AMP- and phorbol ester-inducible DNA element. Nature 323(6086):353–356
Commons KG, van Bockstaele EJ, Pfaff DW (1999) Frequent colocalization of mu opioid and NMDA-type glutamate receptors at postsynaptic sites in periaqueductal gray neurons. J Com Neurol 408(4):549–559
Connor M, Bagley EE, Chieng BC, Christie MJ (2015) β-Arrestin-2 knockout prevents development of cellular μ-opioid receptor tolerance but does not affect opioid-withdrawal-related adaptations in single PAG neurons. Br J Pharmacol 172:492–500
Cox BM, Ginsburg M, Osman OH (1968) Acute tolerance to narcotic analgesic drugs in rats. Br J Pharmacol Chemother 33(2):245–256
Cui Y, Chen Y, Zhi J-L, Guo R-X, Feng J-Q, Chen P-X (2006) Activation of p38 mitogen-activated protein kinase in spinal microglia mediates morphine antinociceptive tolerance. Brain Res 1069(1):235–243
Cui Y, Liao X-X, Liu W, Guo R-X, Wu Z-Z, Zhao C-M, Chen P-X, Feng J-Q (2008) A novel role of minocycline: attenuating morphine antinociceptive tolerance by inhibition of p38 MAPK in the activated spinal microglia. Brain Behav Immun 22(1):114–123
Cury Y, Picolo G, Gutierrez VP, Ferreira SH (2011) Pain and analgesia: the dual effect of nitric oxide in the nociceptive system. Nitric Oxide 25:243–254
Dambisya YM, Lee T-L (1996) Role of nitric oxide in the induction and expression of morphine tolerance and dependence in mice. Br J Pharmacol 117(5):914–918
Dang VC, Williams JT (2005) Morphine-induced mu-opioid receptor desensitization. Mol Pharmacol 68(4):1127–1132
De Gortari P, Mengod G (2010) Dopamine D1, D2 and mu-opioid receptors are co-expressed with adenylyl cyclase 5 and phosphodiesterase 7B mRNAs in striatal rat cells. Brain Res 1310:37–45
Dickenson AH, Sullivan AF (1987) Evidence for a role of the NMDA receptor in the frequency dependent potentiation of deep rat dorsal horn nociceptive neurones following C fibre stimulation. Neuropharmacol 26(8):1235–1238
Doll C, Konietzko J, Poll F, Koch T, Hollt V, Schulz S (2011) Agonist-selective patterns of μ-opioid receptor phosphorylation revealed by phosphosite-specific antibodies. Br J Pharmacol 164(2):298–307
Durmus N, Bagcivan I, Ozdemir E, Altun A, Gursoy S (2014) Soluble guanylyl cyclase activators increase expression of tolerance to morphine analgesic effect. Bratisl Lek Listy 115(6):334–339
El Kouhen R, Burd AL, Erickson-Herbrandson LJ, Chang C-Y, Law P-Y, Loh HH (2001) Phosphorylation of Ser363, Thr370, and Ser375 residues within the carboxyl tail differentially regulates μ-opioid receptor internalization. J Biol Chem 276(16):12774–12780
Elliott K, Minami N, Kolesnikov YA, Pasternak GW, Inturrisi CE (1994) The NMDA receptor antagonists, LY274614 and MK-801, and the nitric oxide synthase inhibitor, NG-nitro-L-arginine, attenuate analgesic tolerance to the mu-opioid morphine but not to kappa opioids. Pain 56(1):69–75
Eppler CM, Hulmes JC, Wang JB, Johnson B, Corbett M, Luthin DR, Uhl GR, Linden J (1993) Purification and partial amino acid sequence of a μ opioid receptor from rat brain. J Biol Chem 268(35):26447–26451
Fairbanks CA, Wilcox GL (2000) Spinal plasticity of acute opioid tolerance. J Biomed Sci 7(3):200–212
Fairbanks CA, Wilcox GL (1997) Acute tolerance to spinally administered morphine compares mechanistically with chronically induced morphine tolerance. J Pharmacol Exp Ther 282(3):1408–1417
Fan XL, Zhang JS, Zhang XQ, Yue W, Ma L (2003) Differential regulation of β-arrestin 1 and β-arrestin 2 gene expression in rat brain by morphine. Neurosci 117(2):383–389
Feng B, Li Z, Wang JB (2011) Protein kinase C-mediated phosphorylation of the μ-opioid receptor and its effects on receptor signaling. Mol Pharmacol 79:768–775
Fen X, Zhang J, Zhang X, Yue W, Ma L (2002) Acute and chronic morphine treatments and morphine withdrawal differentially regulate GRK2 and GRK5 gene expression in rat brain. Neuropharmacology 43(3):809–816
Ferguson SSG (2001) Evolving concepts in G-protein coupled endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev 53:1–24
Ferrer-Alcón M, Cardia-Fuster MJ, La Harpe R, Garcia-Sevilla JA (2004) Long-term regulation of signalling components of adenylyl cyclase and mitogen-activated protein kinase in the pre-frontal cortex of human opiate addicts. J Neurochem 90:220–230
Finn AK, Whistler JL (2001) Endocytosis of the mu opioid receptor reduces tolerance and a cellular hallmark of opiate withdrawal. Neuron 32(5):829–839
Förstermann U, Closs EI, Pollock JS, Nakane M, Schwarz P, Gath I, Kleinert H (1994) Nitric oxide synthase isozymes. Characterization, purification, molecular cloning, and function. Hypertension 23(2):1121–1131
Fundytus ME, Coderre TJ (1996) Chronic inhibition of intracellular Ca2+ release or protein kinase C activation significantly reduces the development of morphine dependence. Eur J Pharmacol 300:173–181
Garzón J, Rodríguez-Muñoz M, Sánchez-Blázquez P (2005a) Morphine alters the selective association between mu-opioid receptors and specific RGS proteins in mouse periaqueductal gray matter. Neuropharmacology 48(6):853–868
Garzón J, Rodríguez-Muñoz M, López-Fando A, Sánchez-Blázquez P (2005b) The RGSZ2 protein exists in a complex with μ-opioid receptors and regulates the desensitizing capacity of Gz proteins. Neuropsychopharmacology 30(9):1632
Garzón J, Rodríguez-Muñoz M, López-Fando A, Sánchez-Blázquez P (2005c) Activation of μ-opioid receptors transfers control of Gα subunits to the regulator of G-protein signaling RGS9-2 role in receptor desensitization. J Biol Chem 280(10):8951–8960
Garzón J, Rodríguez-Muñoz M, Vicente-Sánchez A, Bailón C, Martínez-Murillo R, Sánchez-Blázquez P (2011) RGSZ2 binds to the neural nitric oxide synthase PDZ domain to regulate mu-opioid receptor-mediated potentiation of the N-methyl-D-aspartate receptor-calmodulin-dependent protein kinase II pathway. Antioxid Redox Signal 15(4):873–887
Garzón J, Rodriguez- Muñoz M, Sánchez-Blazquez P (2012) Direct association of mu-opioid and NMDA glutamate receptors supports their cross-regulation: molecular implications for opioid tolerance. Curr Drug Abuse Rev 5:199–226
Georges F, Stinus L, Le Moine C (2000) Mapping of c-fos gene expression in the brain during morphine dependence and precipitated withdrawal, and phenotypic identification of the striatal neurons involved. Eur J Neurosci 12(12):4475–4486
Glass MJ, Vanyo L, Quimson L, Pickel VM (2009) Ultrastructural relationship between N-methyl-D-aspartate-NR1 receptor subunit and mu-opioid receptor in the mouse central nucleus of the amygdala. Neuroscience 163(3):857–867
Gracy KN, Svingos AL, Pickel VM (1997) Dual ultrastructural localization of μ-opioid receptors and NMDA-type glutamate receptors in the shell of the nucleus accumbens. J Neurosci 17(12):4839–4848
Granados-Soto V, Kalcheva I, Hua X-Y, Newton A, Yaksh TL (2000) Spinal PKC activity and expression: role in tolerance produced by continuous spinal morphine infusion. Pain 85(3):395–404
Grassi C, Dascenzo M, Azzena GB (2004) Modulation of Cav1 and Cav2.2 channels induced by nitric oxide via cGMP-dependent protein kinase. Neurochem Int 45(6):885–893
Guang W, Wang H, Su T, Weinstein IB, Wang JB (2004) Role of mPKCI, a novel μ-opioid receptor interactive protein, in receptor desensitization, phosphorylation, and morphine-induced analgesia. Mol Pharmacol 66:1285–1292
Guitart X, Thompson MA, Mirante CK, Greenberg ME, Nestler EJ (1992) Regulation of cyclic AMP response element binding protein (CREB) phosphorylation by acute and chronic morphine in the rat locus coeruleus. J Neurochem 58(3):1168–1171
Hanoune J, Defer N (2001) Regulation and role of adenylyl cyclase isoforms. Ann Rev Pharmacol Toxicol 41:145–174
Han M-H, Bolaños CA, Green TA, Olson VG, Neve RL, Liu R-J, Aghajanian GK, Nestler EJ (2006) Role of cAMP response element-binding protein in the rat locus ceruleus: regulation of neuronal activity and opiate withdrawal behaviors. J Neurosci 26(17):4624–4629
Harris GC, Williams JT (1991) Transient homologous μ-opioid receptor desensitization in rate locus coeruleus neurons. J Neurosci 11(8):2574–2581
Hassan HE, Myers AL, Lee IJ, Coop A, Eddington ND (2007) Oxycodone induces overexpression of P-glycoprotein (ABCB1) and affects paclitaxel’s tissue distribution in Sprague Dawley rats. J Pharmaceut Sci 96(9):2494–2506
Heinzen EL, Pollack GM (2004) The development of morphine antinociceptive tolerance in nitric oxide synthase-deficient mice. Biochem Pharmacol 67(4):735–741
Heinzen EL, Booth RG, Pollack GM (2005) Neuronal nitric oxide modulates morphine antinociceptive tolerance by enhancing constitutive activity of the μ-opioid receptor. Biochem Pharmacol 69(4):679–688
Hervera A, Leánez S, Pol O (2012) The inhibition of the nitric oxid-cGMP-PKG-JNK signaling pathway avoids the development of tolerance to the local antiallodynic effects produced by morphine during neuropathic pain. Eur J Pharmacol 685(1–3):42–51
He L, Kim JA, Whistler JL (2009) Biomarkers of morphine tolerance and dependence are prevented by morphine-induced endocytosis of a mutant μ-opioid receptor. FASEB J 23(12):4327–4334
He X-T, Zhou K-X, Zhao W-J, Zhang C, Deng J-P, Chen F-M, Gu Z-X, Li Y-Q, Dong Y-L (2018) Inhibition of histone deacetylases attenuates morphine tolerance and restores MOR expression in the DRG of BCP rats. Front Pharmacol 9:509
Hong S, Zhao L, Qu Y, Li Z, Zhao Y, Li L (2009) Morphine-induced changes in adenylate and guanylate cyclase in locus ceruleus, periaqueductal gray, and substantia nigra in rats. Am J Drug Alcohol Abuse 35(3):133–137
Horvath RJ, Romero-Sandoval EA, De Leo JA (2010) Inhibition of microglial P2X4 receptors attenuates morphine tolerance, Iba1, GFAP and mu opioid receptor protein expression while enhancing perivascular microglial ED2. Pain 150(4):401–413
Hoskins B, Ho IK, Meydrech EF (1985) Effects of aging and morphine administration on calmodulin and calmodulin-regulated enzymes in striata of mice. J Neurochem 44(4):1069–1073
Hoyt KR, Tang L-H, Aizenman E, Reynolds EI (1992) Nitric oxide modulates NMDA-induced increases in intracellular calcium in rat forebrain neurons. Brain Res 592:3120–3316
Ho IK, Loh HH, Way EL (1973) Effect of cyclic 3’,5’-adenosine monophosphate on morphine tolerance and physical dependence. J Pharmacol Exp Ther 185(2):347–357
Huang M, Luo L, Zhang Y, Wang W, Dong J, Du W, Jiang W, Xu T (2019a) Metabotropic glutamate receptor 5 signalling induced NMDA receptor subunits alterations during the development of morphine-induced antinociceptive tolerance in mouse cortex. Biomed Pharmacother 110:717–726
Huang Y, Hu L, Huang Y, Li Y, Yang J, Gu J, Xu H (2019b) PKA-mediated phosphorylation of CREB and NMDA receptor 2B in the hippocampus of offspring rats involved in transmission of mental disorders across a generation. Psychiatry Res 280:112497
Huidobro-Toro JP, Way EL (1978) Single-dose tolerance to antinociception, and physical dependence on β-endorphin in mice. Eur J Pharmacol 52(2):179–189
Huidobro F, Huidobro-Toro JP, Way EL (1976) Studies on tolerance development to single doses of morphine in mice. J Pharmacol Exp Ther 198(2):318–329
Hunt SP, Pini A, Evan G (1987) Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature 328(6131):632–634
Inturrisi CE (2002) Clinical pharmacology of opioids for pain. Clin J Pain 18(Suppl 4):S3–S13
Jiang Z, Wu S, Wu X, Zhong J, Liv A, Jiao J, Chen Z (2016) Blocking mammalian target of rapamycin alleviates bone cancer pain and morphine tolerance via μ-opioid receptor. Int J Cancer 138:2103–2020
Johnson PS, Wang JB, Wang WF, Uhl GR (1994) Expressed mu opiate receptor couples to adenylate cyclase and phosphatidyl inositol turnover. NeuroReport 5(4):507–509
Kach J, Sethakorn N, Dulin NO (2012) A finer tuning of G-protein signaling through regulated control of RGS proteins. Am J Physiol Heart Circ Physiol 303(1):H19–H35
Karoussiotis C, Marti-Solano M, Stepniewski TM, Symeonof A, Selent J, Georgoussi Z (2020) A highly conserved δ-opioid receptor region determines RGS4 interaction. FEBS J 287(4):736–748
Kaya AI, Perry NA, Gurevich VV, Iverson TM (2020) Phosphorylation barcode-dependent signal bias of the dopamine D1 receptor. Proc Natl Acad Sci USA 117(25):14139–14149
Keith DE, Murray SR, Zaki PA, Chu PC, Lissin DV, Kang L, Evans CJ, von Zastrow M (1996) Morphine activates opioid receptors without causing their rapid internalization. J Biol Chem 271(32):19021–19024
Khattab MM, El-Hadiyah TM, Al-Shabanah OA, Raza M (2004) Modification by L-NAME of codeine induced analgesia: possible role of nitric oxide. Receptors Channels 10(5–6):139–145
Kibaly C, Lin H-Y, Loh HH, Law P-Y (2017) Spinal or supraspinal phosphorylation deficiency at the MOR C-terminus does not affect morphine tolerance in vivo. Pharmacol Res 119:153–168
Kieffer BL, Evans CJ (2002) Opioid tolerance—in search of the Holy Grail. Cell 108:587–590
Kim K-S, Lee K-W, Lee K-W, Im J-Y, Kim S-W, Lee J-K, Nestler EJ, Han P-L (2006) Adenylyl cyclase type 5 (AC5) is an essential mediator of morphine action. Proc Natl Acad Sci USA 103(10):3908–3913
Kim JA, Bartlett S, He L, Nielsen CK, Chang AM, Kharazia V, Waldhoer M, Ou CJ, Taylor S, Ferwerda M, Cado D, Whistler JL (2008) Morphine-induced receptor endocytosis in a novel knockin reduces tolerance and dependence. Curr Biol 18(2):129–135
Kim HJ, Kim H, Jung MH, Kwon YK, Kim BJ (2016) Berberine induces pacemaker potential inhibition via cGMP-dependent ATP-sensitive K+ channels by stimulating mu/delta opioid receptors in cultured interstitial cells of Cajal from mouse small intestine. Mol Med Rep 14(4):3985–3991
King M, Su W, Chang A, Zuckerman A, Pasternak GW (2001) Transport of opioids from the brain to the periphery by P-glycoprotein: peripheral actions of central drugs. Nat Neurosci 4(3):268–274
Klee WA, Nirenberg M (1974) A neuroblastoma x glioma hybrid cell line with morphine receptors. Proc Natl Acad Sci USA 71(9):3474–3477
Kliewer A, Schmiedel F, Sianati S, Bailey A, Bateman JT, Levitt ES, Williams JT, Christie MJ, Schulz S (2019) Phosphorylation-deficient G-protein-biased μ-opioid receptors improve analgesia and diminish tolerance but worsen opioid side effects. Nat Commun 10(1):367
Koch T, Schulz S, Schröder H, Wolf R, Raulf E, Höllt V (1998) Carboxyl-terminal splicing of the rat μ opioid receptor modulates agonist-mediated internalization and receptor resensitization. J Biol Chem 273(22):13652–13657
Kolesnikov Y, Pasternak GW (1999) Topical opioids in mice: analgesia and reversal of tolerance by a topical N-methyl-D-aspartate antagonist. J Pharmacol Exp Ther 290(1):247–252
Kolesnikov YA, Pick CG, Pasternak GW (1992) NG-Nitro-L-arginine prevents morphine tolerance. Eur J Pharmacol 221:399–400
Kolesnikov YA, Ferkany J, Pasternak GW (1993a) Blockade of mu and kappa1 opioid analgesic tolerance by NPC17742, a novel NMDA antagonist. Life Sci 53(19):1489–1494
Kolesnikov YA, Pick CG, Ciszewska G, Pasternak GW (1993b) Blockade of tolerance to morphine but not to K opioids by a nitric oxide synthase inhibitor. Proc Natl Acad Sci USA 90:5162–5166
Kolesnikov YA, Pan YX, Babey AM, Jain S, Wilson R, Pasternak GW (1997) Functionally differentiating two neuronal nitric oxide synthase isoforms through antisense mapping: evidence for opposing NO actions on morphine analgesia and tolerance. Proc Nat Acad Sci USA 94(15):8220–8225
Kolesnikov YA, Chereshnev I, Criesta M, Pan YX, Pasternak GW (2009) Opposing actions of neuronal nitric oxide synthase isoforms in formalin-induced pain in mice. Brain Res 1289:14–21
Kovoor A, Celver JP, Wu A, Chavkin C (1998) Agonist induced homologous desensitization of mu-opioid receptors mediated by G protein-coupled receptor kinases is dependent on agonist efficacy. Mol Pharmacol 54(4):704–711
Kramer HK, Simon EJ (1999a) Role of protein kinase C (PKC) in agonist-induced receptor down-regulation: I: PKC translocation to the membrane of SH-SY5Y neuroblastoma cells is induced by μ-opioid agonists. J Neurochem 72(2):585–593
Kramer HK, Simon EJ (1999b) Role of protein kinase C (PKC) in agonist-induced receptor down-regulation: II. Activation and involvement of the α, δ and ζ isoforms of PKC. J Neurochem 72(2):594–604
Kritikos PG, Papadaki SP (1967) The history of the poppy and of opium and their expansion in antiquity in the eastern Mediterranean area. Bull Narc 3:17–38
Kuo JF, Greengard P (1969) Cyclic nucleotide-dependent protein kinases, IV. Widespread occurrence of adenosine 3’,5’-monophosphate-dependent protein kinase in various tissues and phyla of the animal kingdom. Proc Natl Acad Sci USA 64(4):1349–1355
Kuriyama K, Nakagawa K, Naito K, Muramatsu M (1978) Morphine-induced changes in cyclic AMP metabolism and protein kinase activity in the brain. Jpn J Pharmacol 28(1):73–84
Lane-Ladd SB, Pineda J, Boundy VA, Pfeuffer T, Krupinski J, Aghajanian GK, Nestler EJ (1997) CREB (cAMP response element-binding protein) in the locus coeruleus: biochemical, physiological, and behavioral evidence for a role in opiate dependence. J Neurosci 17(20):7890–7901
Laughlin S, Sejnowski TJ (2003) Communication in neuronal networks. Science 301(5641):1870–1874
Law P-Y, Wong YH, Loh HH (2000) Molecular mechanisms and regulation of opioid receptor signaling. Annu Rev Pharmacol Toxicol 40:389–430
Lee I-O, Yukhananov R, Standaert DG, Crosby G (2004) NMDA-R1 antisense oligodeoxynucleotides modify formalin-induced nociception and spinal c-Fos expression in rat spinal cord. Pharmacol Biochem Behav 79(1):183–188
Leff ER, Arttamangkul S, Williams JT (2020) Chronic treatment with morphine disrupts acute kinase-dependent desensitization of GPCRs. Mol Pharmacol 98(4):497–507
Lefkowitz RJ (1993) G-protein receptor kinases. Cell 74(3):409–412
Li X, Clark JD (1999) Morphine tolerance and transcription factor expression in mouse spinal cord tissue. Neurosci Lett 272(2):79–82
Lin Y, Jover-Mengual T, Wong J, Bennett MVL, Zukin RS (2006) PSD-95 and PKC converge in regulating NMDA receptor trafficking and gating. Proc Natl Acad Sci USA 103(52):19902–19907
Liu J, Nickolenko J, Sharp FR (1994) Morphine induces c-fos and junB in striatum and nucleus accumbens via D1 and N-methyl-d-aspartate receptors. Proc Natl Acad Sci USA 91(18):8537–8541
Liu Q, Puche AC, Wang JB (2008) Distribution and expression of protein kinase C interacting protein (PKCI/HINT1) in mouse central nervous system. Neurochem Res 33(7):1263–1276
Liu P, Liu Z, Wang J, Ma X, Dang Y (2017) HINT1 in neuropsychiatric disease: a potential neuroplastic mediator. Neural Plast 2017:5181925
Llorente J, Santamarta MT, Henderson G, Pineda J (2012) Enhancement of μ-opioid receptor desensitization by nitric oxide in rat locus coeruleus neurons: involvement of reactive oxygen species. J Pharmacol Exp Ther 342:552–560
Loh HH, Shen FH, Way EL (1969) Inhibition of morphine tolerance and physical dependence development and serotonin synthesis by cycloheximide. Biochem Pharmacol 18(12):2711–2721
Lotti VJ, Lomax P, George R (1966) Acute tolerance to morphine following systemic and intracerebral injection in the rat. Int J Neuropharmacol 5(1):35–42
Luger TJ, Lorenz IH, Grabner-Weiss C, Hayashi T (1995) Effect of the NMDA-antagonist, MK 801, on benzodiazepine-opioid interactions at the spinal and supraspinal level in rats. Br J Pharmacol 114(5):1097–1103
Lutfy K, Hurlbut DE, Weber E (1993) Blockade of morphine-induced analgesia and tolerance in mice by MK-801. Brain Res 616(1–2):83–88
Lutz BM, Nia S, Xiong M, Tao Y-X, Bekker A (2015) mTOR, a new potential target for chronic pain and opioid-induced tolerance and hyperalgesia. Mol Pain 11:32
Lu W-Y, Xiong Z-G, Lei S, Orser BA, Dudek E, Browning MD, MacDonald JF (1999) G-protein-coupled receptors act via protein kinase C and Src to regulate NMDA receptors. Nat Neurosci 2(4):331–338
Lu Z, Xu J, Xu M, Pasternak GW, Pan Y-X (2014) Morphine regulates expression of μ-opioid receptor MOR-1A, an intron-retention carboxy terminal splice variant of the μ-opioid receptor (OPRM1) gene via miR-103/miR-107. Mol Pharmacol 85(2):368–380
Lysle DT, Carrigan KA (2001) Morphine-6β-glucuronide modulates the expression of inducible nitric oxide synthase. Inflammation 25(4):267–275
Machelska H, Ziólkowska B, Mika J, Przewlocka B, Przewlocki R (1997) Chronic morphine increases biosynthesis of nitric oxide synthase in the rat spinal cord. NeuroReport 8(12):2743–2747
Macht DI (1915) The history of opium and some of its preparations and alkaloids. JAMA 11(6):477–481
Maduna T, Audouard E, Dembélé D, Mouzaoui N, Reiss D, Massotte D, Gaveriaux-Ruff C (2019) Microglia express mu opioid receptor: insights from transcriptomes and fluorescent reporter mice. Front Psychiatry 9:726
Majeed NH, Przewlocka B, Machelska H, Przewlocki R (1994) Inhibition of nitric oxide attenuates the development of morphine tolerance and dependence in mice. Neuropharmacology 33(2):189–192
Mann A, Liebetrau S, Klima M, Dasgupta P, Massotte D, Schulz S (2020) Agonist-induced phosphorylation bar code and differential post-activation signaling of the delta opioid receptor revealed by phosphosite-specific antibodies. Sci Rep 10(1):8585
Manning BH, Mao J, Frenk H, Price DD, Mayer DJ (1996) Continuous co-administration of dextromethorphan or MK-801 with morphine: attenuation of morphine dependence and naloxone-reversible attenuation of morphine tolerance. Pain 67(1):79–88
Mann A, Illing S, Miess E, Schulz S (2015) Different mechanisms of homologous and heterologous μ-opioid receptor phosphorylation. Br J Pharmacol 172(2):311–316
Mansouri MT, Naghizadeh B, Ghorbanzadeh B, Alboghobeish S, Amirgholami N, Houshmand G, Cauli O (2018) Venlafaxine prevents morphine antinociceptive tolerance: the role of neuroinflammation and the l-arginine-nitric oxide pathway. Exp Neurol 303:134–141
Mao J, Sung B, Ji R-R, Lim G (2002) Chronic morphine induces downregulation of spinal glutamate transporters: implications in morphine tolerance and abnormal pain sensitivity. J Neurosci 22(18):8312–8323
Marek P, Ben-Eliyahu S, Gold M, Liebeskind JC (1991a) Excitatory amino acid antagonists (kynurenic acid and MK-801) attenuate the development of morphine tolerance in the rat. Brain Res 547(1):77–81
Marek P, Ben-Eliyahu S, Vaccarino AL, Liebeskind JC (1991b) Delayed application of MK-801 attenuates development of morphine tolerance in rats. Brain Res 558(1):163–165
Marrone GF, Le Rouzic V, Varadi A, Xu J, Rajadhyaksha AM, Majumdar S, Pan Y-X, Pasternak GW (2017) Genetic dissociation of morphine analgesia from hyperalgesia in mice. Psychopharmacology 234(12):1891–1900
Mehalick ML, Ingram SL, Aicher SA, Morgan MM (2013) Chronic inflammatory pain prevents tolerance to the antinociceptive effect of morphine microinjected into the ventrolateral periaqueductal gray of the rat. J Pain 14(12):1601–1610
Mendell LM (1966) Physiological properties of unmyelinated fiber projection to the spinal cord. Exp Neurol 16(3):316–332
Mendell LM, Wall PD (1965) Responses of single dorsal cord cells to peripheral cutaneous unmyelinated fibres. Nature 206(4979):97–99
Mercadante S, Fulfaro F, Casuccio A (2002) A randomised controlled study on the use of anti-inflammatory drugs in patients with cancer pain on morphine therapy: effects on dose-escalation and a pharmacoeconomic analysis. Eur J Cancer 38(10):1358–1363
Merighi S, Gessi S, Varani K, Fazzi D, Stephanelli A, Borea PA (2013) Morphine mediates a proinflammatory phenotype via μ-opioid receptor-PKCe-Akt-ERK1/2 signaling pathway in activated microglial cells. Biochem Pharmacol 86(4):487–496
Miyamoto E, Kuo JF, Greengard P (1969a) Adenosine 3’,5’-monophosphate-dependent protein kinase from brain. Science 165(3888):63–65
Miyamoto E, Kuo JF, Greengard P (1969b) Cyclic nucleotide-dependent protein kinases III. Purification and properties of adenosine 3’,5’-monophosphate-dependent protein kinase from bovine brain. J Biol Chem 244(23):6395–6402
Montminy MR, Bilezikjian LM (1987) Binding of a nuclear protein to the cyclic-AMP response element in the somatostatin gene. Nature 328(6126):175–178
Mousa SA, Shaqura M, Winkler J, Khalefa BI, Al-Madol MA, Shakibaei M, Schulz S, Schäfer M (2016) Protein kinase C-mediated mu-opioid receptor phosphorylation and desensitization in rats, and its prevention during early diabetes. Pain 157(4):910–921
Murray F, Harrison NJ, Grimwood S, Bristow LJ, Hutson PH (2007) Nucleus accumbens NMDA receptor subunit expression and function is enhanced in morphine-dependent rats. Eur J Pharmacol 562(3):191–197
Murthy KS, Makhlouf GM (1996) Opioid μ, δ, and κ receptor-induced activation of phospholipase C-β 3 and inhibition of adenylyl cyclase is mediated by Gi2 and G0 in smooth muscle. Mol Pharmacol 50(4):870–877
Naidu P, Singh A, Joshi D, Kulkarni S (2003) Possible mechanisms of action in quercetin reversal of morphine tolerance and dependence. Addict Biol 8(3):327–336
Nestler EJ, Alreja M, Aghajanian GK (1994) Molecular and cellular mechanisms. Of opiate action: studies in the rat locus coeruleus. Brain Res Bull 35(5–6):521–528
Nestler EJ, Aghajanian GK (1997) Molecular and cellular basis of addiction. Science 278(5335):58–63
Núñez C, Martín F, Földes A, Laorden ML, Kovács KJ, Milanés MV (2010) Induction of FosB/ΔFosB in the brain stress system-related structures during morphine dependence and withdrawal. J Neurochem 114:475–487
Ozdemir E, Bagcivan I, Durmus N, Altun A, Gursoy S (2011) The nitric oxide–cGMP signalling pathway plays a significant role in tolerance to the analgesic effect of morphine. Can J Physiol Pharmacol 89(2):89–95
Paez Espinosa V, Liu Y, Ferrini M, Anghel A, Nie Y, Tripathi PV, Porche R, Jansen E, Stuart RC, Nillni EA, Lutfy K, Friedman TC (2008) Differential regulation of prohormone convertase 1/3, prohormone convertase 2 and phosphorylated cyclic-AMP-response element binding protein by short-term and long-term morphine treatment: implications for understanding the “switch” to opiate addiction. Neurosci 156:788–799
Pan Y-X (2005) Diversity and complexity of the mu opioid receptor gene: alternative pre-mRNA splicing and promotors. DNA Cell Biol 24(11):736–750
Pan Y-X, Xu J, Xu M, Rossi GC, Matulonis JE, Paternak GW (2009) Involvement of exon 11-associated variants of the mu opioid receptor MOR-1 in heroin, but not morphine, actions. Proc Natl Acad Sci USA 106(12):4917–4922
Pan Y-X, Xu J, Bolan E, Abbadie C, Chang A, Zuckerman A, Rossi C, Pasternak GW (1999) Identification and characterization of three new alternatively spliced μ-opioid receptor isoforms. Mol Pharmacol 56(2):396–403
Pan Y-X, Xu J, Bolan E, Chang A, Mahurter LA, Rossi G, Pasternak GW (2000) Isolation and expression of a novel alternatively spliced mu opioid receptor isoform, MOR-1F. FEBS Lett 466(2–3):337–340
Parsons MP, Raymond LA (2014) Extrasynaptic NMDA receptor involvement in central nervous system disorders. Neuron 82(2):279–293
Pasternak GW (1995) Nitric oxide and opioid tolerance. NIDA Res Monogr 147:182–194
Pasternak GW (2007) When it comes to opiates, just say NO. J Clin Invest 117(11):3185–3187
Pasternak GW, Inturrisi CE (1995) Pharmacological modulation of opioid tolerance. Exp Opin Invest Drugs 4(4):271–281
Pasternak GW, Kolesnikov Y, Babey AM (1995) Perspectives on the N-methyl-d-aspartate/nitric oxide cascade and opioid tolerance. Neuropsychopharmacol 13:309–313
Pasternak GW, Pan Y-X (2013) Mu opioids and their receptors: evolution of a concept. Pharmacol Rev 65(4):1257–1317
Pert CB, Snyder SH (1973) Opiate receptor: demonstration in nervous tissue. Science 179(4077):1011–1014
Popiolek-Barczyk K, Piotrowska A, Makuch W, Mika J (2017) Biphalin, a dimeric enkephalin, alleviates LPS-induced activation of rat primary microglial cultures in opioid receptor-dependent and receptor-independent manners. Neural Plast 2017:3829472
Portenoy RK (2000) Current pharmacotherapy of chronic pain. J Pain Symptom Manage 19(1 Suppl):S16–S20
Reiss D, Maduna T, Maurin H, Audouard E, Gaveriaux-Ruff C (2020) Mu opioid receptor in microglia contributes to morphine analgesic tolerance, hyperalgesia and withdrawal in mice. J Neurosci Res. https://doi.org/10.1002/jnr.24626
Ren KE, Hylden JL, Williams GM, Ruda MA, Dubner R (1992) The effects of a non-competitive NMDA receptor antagonist, MK-801, on behavioral hyperalgesia and dorsal horn neuronal activity in rats with unilateral inflammation. Pain 50(3):331–344
Ribeiro FM, Vieira LB, Pires RGW, Olmo RP, Ferguson SSG (2017) Metabotropic glutamate receptors and neurodegenerative diseases. Pharmacol Res 115:179–191
Rodríguez-Muñoz M, Garzón J (2013) Nitric oxide and zinc-mediated protein assemblies involved in mu opioid receptor signaling. Mol Neurobiol 48(3):769–782
Rodríguez-Muñoz M, de la Torre-Madrid E, Sánchez-Blázquez P, Garzón J (2007b) Morphine induces endocytosis of neuronal μ-opioid receptors through the sustained transfer of Gα subunits to RGSZ2 proteins. Mol Pain 3(1):19
Rodríguez-Muñoz M, de la Torre-Madrid E, Gaitán G, Sánchez-Blázquez P, Garzón J (2007a) RGS14 prevents morphine from internalizing Mu-opioid receptors in periaqueductal gray neurons. Cell Signal 19(12):2558–2571
Rodríguez-Muñoz M, de la Torre-Madrid E, Sánchez-Blázquez P, Wang JB, Garzón J (2008) NMDAR-nNOS generated zinc recruits PKCγ to the HINT1–RGS17 complex bound to the C terminus of Mu-opioid receptors. Cell Signal 20(10):1855–1864
Rodríguez-Muñoz M, de la Torre-Madrid E, Sánchez-Blázquez P, Garzón J (2011) NO-released zinc supports the simultaneous binding of Raf-1 and PKCγ cysteine-rich domains to HINT1 protein at the mu-opioid receptor. Antioxid Redox Signal 14(12):2413–2425
Roeckel L-A, Le Coz G-M, Gaveriaux-Ruff C, Simonin F (2016) Opioid-induced hyperalgesia: cellular and molecular mechanisms. Neuroscience 338:160–182
Roesler WJ, Vandenbark GR, Hanson RW (1988) Cyclic AMP and the induction of eukaryotic gene transcription. J Biol Chem 263(19):9063–9066
Rosenfeld GC, Burks TF (1977) Single-dose tolerance to morphine hypothermia in the rat: differentiation of acute from long-term tolerance. J Pharmacol Exp Ther 202(3):654–659
Rossi GC, Pan Y-X, Brown GP, Pasternak GW (1995) Antisense mapping the MOR-1 opioid receptor: evidence for alternative splicing and a novel morphine-6β-glucuronide receptor. FEBS Lett 369(2–3):192–196
Rossi GC, Leventhal L, Pan Y-X, Cole J, Su W, Bodnar RJ, Pasternak GW (1997) Antisense mapping of MOR-1 in rats: distinguishing between morphine and morphine-6β-glucuronide antinociception. J Pharmacol Exp Ther 281(1):109–114
Rubovitch V, Gafni M, Sarne Y (2003) The mu opioid agonist DAMGO stimulates cAMP production in SK-N-SH cells through a PLC–PKC–Ca++ pathway. Mol Brain Res 110(2):261–266
Sadana R, Dessauer CW (2009) Physiological roles for G protein-regulated adenylyl cyclase isoforms: insights from knockout and overexpression studies. Neurosignals 17(1):5–22
Salehi A, Alembizar F, Hosseinkhani A (2016) Anesthesia and pain management in traditional Iranian medicine. Acta Med His Adriat 14(2):317–326
Salvemini D, Neumann W (2010) Targeting peroxynitrite driven nitroxidative stress with synzymes: a novel therapeutic approach in chronic pain management. Life Sci 86(15–16):604–614
Salvemini D, Little JW, Doyle T, Neumann WL (2011) Roles of reactive oxygen and nitrogen species in pain. Free Radic Biol Med 51(5):951–966
Sánchez-Blázquez P, Rodríguez-Muñoz M, Garzón J (2010) μ-opioid receptors transiently activate the Akt-nNOS pathway to produce sustained potentiation of PKC-mediated NMDAR-CaMKII signaling. PLoS ONE 5(6):e11278
Sanna MD, Borgonetti V, Galeotti N (2019) m Opioid receptor-triggered Notch-1 activation contributes to morphine tolerance: role for neuron-glia communication. Mol Neurobiol 57(1):331–345
Santamarta MT, Llorente J, Mendiguren A, Pineda J (2014) Involvement of neuronal nitric oxide synthase in desensitisation of µ-opioid receptors in the rat locus coeruleus. J Psychopharmacol 28(10):903–914
Schaefer CP, Arkwright NB, Jacobs LM, Jarvis CK, Hunn KC, Largent-Milnes TM, Tome ME, Davis TP (2018) Chronic morphine exposure potentiates P-glycoprotein trafficking from nuclear reservoirs in cortical rat brain microvessels. PLoS ONE 13(2):e0192340
Scoto GM, Arico G, Iemolo A, Ronsisvalle G, Parenti C (2010) Selective inhibition of the NOP receptor in the ventrolateral periaqueductal gray attenuates the development and the expression of tolerance to morphine-induced antinociception in rats. Peptides 31:696–700
Senese NB, Kandasamy R, Kochan KE, Traynor JR (2020) Regulator of G protein signaling (RGS) protein modulation of opioid receptor signaling as a possible target for pain management. Front Mol Neurosci 13:5
Sharma SK, Nirenberg M, Klee WA (1975b) Morphine receptors as regulators of adenylate cyclase activity. Proc Natl Acad Sci USA 72(2):590–594
Sharma SK, Klee WA, Nirenberg WA (1975a) Dual regulation of adenylate cyclase accounts for narcotic dependence and tolerance. Proc Nat Acad Sci USA 72(8):3092–3096
Shen J, Gomes B, Gallagher A, Stafford K, Yoburn BC (2000) Role of cAMP-dependent protein kinase (PKA) in opioid agonist-induced μ-opioid receptor downregulation and tolerance in mice. Synapse 38:322–327
Short JM, Wynshaw-Boris A, Short HP, Hanson RW (1986) Characterization of the phosphoenolpyruvate carboxykinase (GTP) promotor-regulatory region II. Identification of cAMP and glucocorticoid regulatory domains. J Biol Chem 261(21):9721–9726
Sibley DR, Strasser RH, Benovic JL, Daniel K, Lefkowitz RJ (1986) Phosphorylation/dephosphorylation of the b-adrenergic receptor regulates its functional coupling to adenylate cyclase and subcellular distribution. Proc Natl Acad Sci USA 83:9408–9412
Simon EJ, Hiller JM, Edelman I (1973) Stereospecific binding of the potent narcotic analgesic [3H]etorphine in rat-brain homogenate. Proc Natl Acad Sci USA 70(7):1947–1949
Simonds WF (1999) G protein regulation of adenylate cyclase. TiPS 20(2):66–72
Sim-Selley LJ, Selley DE, Vogt LJ, Childers SR, Martin TJ (2000) Chronic heroin self-administration desensitizes μ opioid receptor-activated G proteins in specific regions of rat brain. J Neurosci 20(12):4555–4562
Sim LJ, Selley DE, Dworkin SI, Childers SR (1996) Effects of chronic morphine on μ opioid receptor-stimulated [35S]GTPγS autoradiography in rat brain. J Neurosci 16(8):2684–2692
Sloan P, Melzack R (1999) Long-term patterns of morphine dosage and pain intensity among cancer patients. Hosp J 14(2):35–47
Snyder SH, Bredt DS (1991) Nitric oxide as a neuronal messenger. Trends Pharmacol Sci 12:125–128
Stefano GB, Kim E, Liu Y, Zhu W, Casares F, Mantione K, Jones DA, Cadet P (2004) Nitric oxide modulates microglial activation. Med Sci Monit 10(2):BR17-B22
Strijbos PJLM, Leach MJ, Garthwaite J (1996) Vicious cycle involving Na+ channels, glutamate release, and NMDA receptors mediates delayed neurodegeneration through nitric oxide formation. J Neurosci 16(16):5004–5013
Tanowitz M, von Zastrow M (2003) A novel endocytic recycling signal that distinguishes the membrane trafficking of naturally occurring opioid receptors. J Biol Chem 278(46):45978–45986
Terenius L (1973) Characteristics of the “receptor” for narcotic analgesics in synaptic plasma membrane fraction from rat brain. Acta Pharmacol Toxicol 33(5):377–384
Terman GW, Jin W, Cheong Y-P, Lowe J, Caron MG, Lefkowitz RJ, Chavkin C (2004) G-protein receptor kinase 3 (GRK3) influences opioid analgesic tolerance but not opioid withdrawal. Br J Pharmacol 141(1):55–64
Thompson RC, Mansour A, Akil H, Watson SJ (1993) Cloning and pharmacological characterization of a rat μ opioid receptor. Neuron 11(5):903–913
Tiseo PJ, Inturrisi CE (1993) Attenuation and reversal of morphine tolerance by the competitive N-methyl-d-aspartate receptor antagonist LY274614. J Pharmacol Exp Ther 264(3):1090–1096
Tiseo PJ, Cheng J, Pasternak GW, Inturrisi CE (1994) Modulation of morphine tolerance by the competitive N-methyl-D-aspartate receptor antagonist LY274614: assessment of opioid receptor changes. J Pharmacol Exp Ther 268(1):195–201
Toda N, Kishioka S, Hatano Y, Toda H (2009) Modulation of opioid actions by nitric oxide signaling. Anesthesiology 110(1):166–181
Traber J, Fischer K, Latzin S, Hamprecht B (1975a) Morphine antagonizes action of prostaglandin in neuroblastoma and neuroblastoma x glioma hybrid cells. Nature 253(5487):120–122
Traber J, Gullis R, Hamprecht B (1975b) Influence of opiates on the levels of adenosine 3’:5’-cyclic monophosphate in neuroblastoma x glioma hybrid cells. Life Sci 16(12):1863–1868
Trapaidze N, Gomes I, Cvejic S, Bansinath M, Devi LA (2000) Opioid receptor endocytosis and activation of the MAP kinase pathway. Mol Brain Res 76:220–228
Traynor J (2012) μ-Opioid receptors and regulators of G protein signaling (RGS) proteins: from a symposium on new concepts in mu-opioid pharmacology. Drug Alcohol Depend 121(3):173–180
Trujillo KA (2002) The neurobiology of opiate tolerance, dependence and sensitization: mechanisms of NMDA receptor-dependent synaptic plasticity. Neurotoxicol Res 4(4):373–391
Trujillo KA, Akil H (1991) Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801. Science 251(4989):85–87
Tseng LF, Loh HH, Li CH (1977) Human b-endorphin: development of tolerance and behavioral activity in rats. Biochem Biophys Res Commun 74(2):390–396
Turchan-Cholewo J, Dimayuga FO, Ding Q, Keller JN, Hauser KF, Knapp PE, Bruce-Keller AJ (2008) Cell-specific actions of HIV-Tat and morphine on opioid receptor expression in glia. J Neurosci Res 86(9):2100–2110
Ueda H, Inoue M, Matsumoto T (2001) Protein kinase C-mediated inhibition of μ-opioid receptor internalization and its involvement in the development of acute tolerance to peripheral μ-agonist analgesia. J Neurosci 21(9):2967–2973
Ujcíková H, Dlouhá K, Roubalová L, Vosahlíková M, Kagan D (1810) Svoboda P (2011) Up-regulation of adenylylcyclases I and II induced by long-term adaptation of rats to morphine fades away 20 days after morphine withdrawal. Biochim Biophys Acta 12:1220–1229
Vyklicky V, Korinek M, Smejkalova T, Balik A, Krausova B, Kaniakova M, Lichnerova K, Cerny J, Krusek J, Dittert I, Horak M, Vyklicky L (2014) Structure, function, and pharmacology of NMDA receptor channels. Physiol Res 63(Suppl 1):S191–S203
Walsh DA, Perkins JP, Krebs EG (1968) An adenosine 3’,5’-monophosphate-dependent protein kinase from rabbit skeletal muscle. J Biol Chem 243(13):3763–3774
Walwyn W, Evans CJ, Hales TG (2007) β-Arrrestin2 and c-Src regulate constitutive activity and recycling of μ opioid receptors in dorsal root ganglion neurons. J Neurosci 27(19):5092–5104
Wang H-Y, Burns LH (2006) Gβγ that interacts with adenylyl cyclase in opioid tolerance originates from a Gs protein. J Neurobiol 66:1302–1310
Wang JB, Imai Y, Eppler CM, Gregor P, Spivak CE, Uhl GR (1993) m Opiate receptor: cDNA cloning and expression. Proc Natl Acad Sci USA 90(21):10230–10234
Wang ZJ, Wang LX (2006) Phosphorylation: a molecular switch in opioid tolerance. Life Sci 79(18):1681–1691
Wang Y, Guang W, Barbier E, Shapiro P, Wang JB (2007) Mu opioid receptor mutant, T394A, abolishes opioid-mediated adenylyl cyclase superactivation. NeuroReport 18(18):1969–1973
Wang X-F, Barbier E, Chiu Y-T, He Y, Zhan J, Bi G-H, Zhang H-Y, Feng B, Liu-Chen L-Y, Wang JB, Xi Z-X (2016) T394A Mutation at the μ opioid receptor blocks opioid tolerance and increases vulnerability to heroin self-administration in mice. J Neurosci 36(40):10392–10403
Weisman A, Quintner J, Masharawi Y (2019) Congenital insensitivity to pain: a misnomer. J Pain 20(9):1011–1014
Wei E, Loh H (1976) Physical dependence on opiate-like peptides. Science 193(4259):1262–1263
Wen Z-H, Wu G-J, Hsu L-C, Chen W-F, Chen J-Y, Shui H-A, Chou A-K, Wong C-S (2008) N-Methyl-d-aspartate receptor antagonist MK-801 attenuates morphine tolerance and associated glial fibrillary acid protein up-regulation: a proteomic approach. Acta Anaesthesiol Scand 52(4):499–508
Williams JT, Ingram SL, Henderson G, Chavkin C, von Zastrow M, Schulz S, Koch T, Evans CJ, Christie MJ (2013) Regulation of μ-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev 65(1):223–254
Wittwer E, Kern SE (2006) Role of morphine’s metabolites in analgesia: concerns and controversies. AAPS J 8(2):39
Wong CS, Hsu MM, Chou YY, Tao PL, Tung CS (2000) Morphine tolerance increases [3H] MK-801 binding affinity and constitutive neuronal nitric oxide synthase expression in rat spinal cord. Br J Anaesth 85(4):587–591
Xie Z, Li Z, Guo L, Ye C, Li J, Yu X, Yang H, Wang Y, Chen C, Zhang D, Liu-Chen LY (2007) Regulator of G protein signaling proteins differentially modulate signaling of μ and δ opioid receptors. Eur J Pharmacol 565(1–3):45–53
Xu J, Xu M, Rossi GC, Pasternak GW, Pan Y-X (2011) Identification and characterization of seven new exon 11-associated splice variants of the rat mu opioid receptor gene, OPRM1. Mol Pain 7:9
Xu J-T, Zhao J-Y, Zhao X, Ligons D, Tiwari V, Atianjoh FE, Lee C-Y, Liang L, Zang W, Njoku D, Raja SN, Yaster M, Tao Y-X (2014) Opioid receptor-triggered spinal mTORC1 activation contributes to morphine tolerance and hyperalgesia. J Clin Invest 124(2):592–603
Xu J, Faskowitz AJ, Rossi GC, Xu M, Lu Z, Pan Y-X, Pasternak GW (2015) Stabilization of morphine tolerance with long-term dosing: association with selective upregulation of mu-opioid receptor splice variant mRNAs. Proc Nat Acad Sci USA 112(1):279–284
Xu J, Lu Z, Narayan A, Le Rouzic VP, Xu M, Hunkele A, Brown TG, Hoefer WF, Rossi GC, Rice RC, Martinez-Rivera A, Rajadhyaksha AM, Cartegni L, Bassoni DL, Pasternak GW, Pan Y-X (2017) Alternatively spliced mu opioid receptor C termini impact the diverse actions of morphine. J Clin Invest 127(4):1561–1573
Yaksh TL, Onofrio BM (1987) Retrospective consideration of the doses of morphine given intrathecally by chronic infusion in 163 patients by 19 physicians. Pain 31(2):211–223
Yang H-Y, Wu Z-Y, Wood M, Whiteman M, Bian J-S (2014) Hydrogen sulfide attenuates opioid dependence by suppression of adenylate cyclase/cAMP pathway. Antioxid Redox Signal 20(1):31–41
Yan YH, Wang Y, Zhao LX, Jiang S, Loh HH, Law PY, Chen H-Z, Qiu Y (2014) Role of FK506 binding protein 12 in morphine-induced μ-opioid receptor internalization and desensitization. Neurosci Lett 566:231–235
Yoburn BC, Billings B, Duttaroy A (1993) Opioid receptor regulation in mice. J Pharmacol Exp Ther 265(1):314–320
Yu VC, Eiger S, Duan DS, Lameh J, Sadée W (1990) Regulation of cyclic AMP by the mu-opioid receptor in human neuroblastoma SH-SY5Y cells. J Neurochem 55(4):1390–1396
Yu SS, Lefkowitz RJ, Hausdorff WP (1993) b-Adrenergic receptor sequestration. A potential mechanism of receptor resensitization. J Biol Chem 268(1):337–341
Yu Y, Zhang L, Yin X, Sun H, Uhl GR, Wang JB (1997) Mu opioid receptor phosphorylation, desensitization, and ligand efficacy. J Biol Chem 272(46):28869–28874
Zeitz KP, Malmberg AB, Gilbert H, Basbaum AI (2001) Reduced development of tolerance to the analgesic effects of morphine and clonidine in PKCg mutant mice. Pain 94(3):245–253
Zhang Y, Pan Y-X, Kolesnikov Y, Pasternak GW (2006) Immunohistochemical labeling of the mu opioid receptor carboxy terminal splice variant mMOR-1B4 in the mouse central nervous system. Brain Res 1099(1):33–45
Zhang J, Fergusson SSG, Barak LS, Bodduluri S, Laport SA, Law P-Y, Caron MG (1998) Role for G protein-coupled receptor kinase in agonist-specific regulation of μ-opioid receptor responsiveness. Proc Natl Acad Sci USA 95(12):7157–7162
Zhang TJ, Qiu Y, Hua Z (2019) The emerging perspective of morphine tolerance: microRNAs. Pain Res Manag 2019:9432965
Zhang T, Xu J, Pan Y-X (2020) A truncated six transmembrane splice variant MOR-1G. Enhances expression of the full-length seven transmembrane μ-opioid receptor through heterodimerization. Mol Pharmacol 98(4):518–527
Zhao M, Joo DT (2006) Subpopulation of dorsal horn neurons displays enhanced N-methyl-d-aspartate receptor function after chronic morphine exposure. Anesthesiology 104(4):815–825
Zushida K, Kamei J (2002) Effect of MK-801 on the antinociceptive effect of [D-Ala2, N-MePhe4, Glyol-l5]enkephalin in diabetic mice. Eur J Pharmacol 448:39–44
Acknowledgements
The authors would like to thank Matthew West for constructive comments on the manuscript.
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gledhill, L.J., Babey, AM. Synthesis of the Mechanisms of Opioid Tolerance: Do We Still Say NO?. Cell Mol Neurobiol 41, 927–948 (2021). https://doi.org/10.1007/s10571-021-01065-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-021-01065-8