Abstract
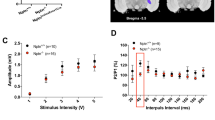
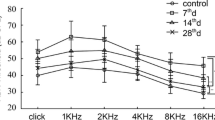
We examined the functional and structural changes of auditory neurons (ANs) in adult mice after conductive hearing loss (CHL). Earplugs (EPs) were bilaterally inserted in male 8-week-old mice for 4 weeks [EP(+) group] and subsequently removed for 4 weeks [EP(+/−) group]. We examined the control mice [EP(−) group] with no EPs inserted at 12 weeks. The auditory brainstem response (ABR) was measured to determine the cochlear function before and after EP insertion, after EP removal, and at 4 weeks following EP removal. We examined the cochleae for hair cell (HC) and spiral ganglion neuron survival, synaptic and neural properties, and AN myelination. There was a significant elevation of the ABR threshold across all tested frequencies after EP insertion. After removing the occlusion, these threshold shifts were fully recovered. Compared with the EP(−) mice, the EP(+) mice showed a significant decrease in the ABR peak 1 amplitude and a significantly prolonged latency at all tested frequencies. There was no significant effect of auditory deprivation on the survival of HCs and ANs. Conversely, auditory deprivation caused significant damage to the synapses and myelin and a significant decrease in the AN size. Although functional changes in the ABR amplitude and latency did not fully recover in the EP(+/−) mice, almost all anatomical changes were fully recovered in the EP(+/−) mice; however, cochlear synapses only showed partial recovery. These results suggest that auditory activities are required to maintain peripheral auditory synapses and myelination in adults. The auditory deprivation model allows for assessment of the mechanisms of synaptopathy and demyelination in the auditory periphery, and synaptic and myelin regeneration in sensorineural hearing loss.






Similar content being viewed by others
References
Aizawa N, Eggermont JJ (2007) Mild noise-induced hearing loss at young age affects temporal modulation transfer functions in adult cat primary auditory cortex. Hear Res 223:71–82
Barclay M, Constable R, James NR, Thorne PR, Montgomery JM (2016) Reduced sensory stimulation alters the molecular make-up of glutamatergic hair cell synapses in the developing cochlea. Neuroscience 325:50–62
Chomiak T, Hu B (2009) What is the optimal value of the g-ratio for myelinated fibers in the rat CNS? A theoretical approach. PLoS ONE 4:e7754
Clarkson C, Antunes FM, Rubio ME (2016) Conductive hearing loss has long-lasting structural and molecular effects on presynaptic and postsynaptic structures of auditory nerve synapses in the cochlear nucleus. J Neurosci 36:10214–10227
Fujimoto C, Yamasoba T (2019) Mitochondria-targeted antioxidants for treatment of hearing loss: a systematic review. Antioxidants 8(4):109
Kim KX, Payne S, Yang-Hood A, Li SZ, Davis B, Carlquist JBVG, Gantz JA, Kallogjeri D, Fitzpatrick JAJ, Ohlemiller KK, Hirose K, Rutherford MA (2019) Vesicular glutamatergic transmission in noise-induced loss and repair of cochlear ribbon synapses. J Neurosci 39:4434–4447
Kujawa SG, Liberman MC (2009) Adding insult to injury: cochlear nerve degeneration after "temporary" noise-induced hearing loss. J Neurosci 29:14077–14085
Kujawa SG, Liberman MC (2015) Synaptopathy in the noise-exposed and aging cochlea: primary neural degeneration in acquired sensorineural hearing loss. Hear Res 330:191–199
Kurioka T, Matsunobu T, Satoh Y, Niwa K, Endo S, Fujioka M, Shiotani A (2015) ERK2 mediates inner hair cell survival and decreases susceptibility to noise-induced hearing loss. Sci Rep 5:16839
Kurioka T, Lee MY, Heeringa AN, Beyer LA, Swiderski DL, Kanicki AC, Kabara LL, Dolan DF, Shore SE, Raphael Y (2016) Selective hair cell ablation and noise exposure lead to different patterns of changes in the cochlea and the cochlear nucleus. Neuroscience 332:242–257
Lassmann H, van Horssen J, Mahad D (2012) Progressive multiple sclerosis: pathology and pathogenesis. Nat Rev Neurol 8:647–656
Lauer AM, Dent ML, Sun W, Xu-Friedman MA (2019) Effects of non-traumatic noise and conductive hearing loss on auditory system function. Neuroscience 407:182–191
Lee DL, Strathmann FG, Gelein R, Walton J, Mayer-Proschel M (2012) Iron deficiency disrupts axon maturation of the developing auditory nerve. J Neurosci 32:5010–5015
Liberman MC, Liberman LD, Maison SF (2015) Chronic conductive hearing loss leads to cochlear degeneration. PLoS ONE 10:e0142341
Liu J, Dietz K, DeLoyht JM, Pedre X, Kelkar D, Kaur J, Vialou V, Lobo MK, Dietz DM, Nestler EJ, Dupree J, Casaccia P (2012) Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat Neurosci 15:1621–1623
Liu K, Jiang X, Shi C, Shi L, Yang B, Shi L, Xu Y, Yang W, Yang S (2013) Cochlear inner hair cell ribbon synapse is the primary target of ototoxic aminoglycoside stimuli. Mol Neurobiol 48:647–654
Mendoza E, Miranda-Barrientos JA, Vazquez-Roque RA, Morales-Herrera E, Ruelas A, De la Rosa G, Flores G, Hernandez-Echeagaray E (2014) In vivo mitochondrial inhibition alters corticostriatal synaptic function and the modulatory effects of neurotrophins. Neuroscience 280:156–170
Naganuma H, Kawahara K, Tokumasu K, Satoh R, Okamoto M (2014) Effects of arginine vasopressin on auditory brainstem response and cochlear morphology in rats. Auris Nasus Larynx 41:249–254
Nelson KR, Gilmore RL, Massey A (1988) Acoustic nerve conduction abnormalities in Guillain-Barre syndrome. Neurology 38:1263–1266
Prendergast G, Tu W, Guest H, Millman RE, Kluk K, Couth S, Munro KJ, Plack CJ (2018) Supra-threshold auditory brainstem response amplitudes in humans: test-retest reliability, electrode montage and noise exposure. Hear Res 364:38–47
Puel JL, Pujol R, Tribillac F, Ladrech S, Eybalin M (1994) Excitatory amino acid antagonists protect cochlear auditory neurons from excitotoxicity. J Comp Neurol 341:241–256
Pujol R, Lenoir M, Robertson D, Eybalin M, Johnstone BM (1985) Kainic acid selectively alters auditory dendrites connected with cochlear inner hair cells. Hear Res 18:145–151
Qi Y, Yu S, Du Z, Qu T, He L, Xiong W, Wei W, Liu K, Gong S (2019) Long-term conductive auditory deprivation during early development causes irreversible hearing impairment and cochlear synaptic disruption. Neuroscience 406:345–355
Richardson RT, O'Leary S, Wise A, Hardman J, Clark G (2005) A single dose of neurotrophin-3 to the cochlea surrounds spiral ganglion neurons and provides trophic support. Hear Res 204:37–47
Schimmang T, Tan J, Muller M, Zimmermann U, Rohbock K, Kopschall I, Limberger A, Minichiello L, Knipper M (2003) Lack of Bdnf and TrkB signalling in the postnatal cochlea leads to a spatial reshaping of innervation along the tonotopic axis and hearing loss. Development 130:4741–4750
Shepherd RK, Colreavy MP (2004) Surface microstructure of the perilymphatic space: implications for cochlear implants and cell- or drug-based therapies. Arch Otolaryngol Head Neck Surg 130:518–523
Sinclair JL, Fischl MJ, Alexandrova O, Hebeta M, Grothe B, Leibold C, Kopp-Scheinpflug C (2017) Sound-evoked activity influences myelination of brainstem axons in the trapezoid body. J Neurosci 37:8239–8255
Starr A, Michalewski HJ, Zeng FG, Fujikawa-Brooks S, Linthicum F, Kim CS, Winnier D, Keats B (2003) Pathology and physiology of auditory neuropathy with a novel mutation in the MPZ gene (Tyr145-%3eSer). Brain 126:1604–1619
Tagoe T, Barker M, Jones A, Allcock N, Hamann M (2014) Auditory nerve perinodal dysmyelination in noise-induced hearing loss. J Neurosci 34:2684–2688
van Loon MC, Ramekers D, Agterberg MJ, de Groot JC, Grolman W, Klis SF, Versnel H (2013) Spiral ganglion cell morphology in guinea pigs after deafening and neurotrophic treatment. Hear Res 298:17–26
Wan G, Gomez-Casati ME, Gigliello AR, Liberman MC, Corfas G (2014) Neurotrophin-3 regulates ribbon synapse density in the cochlea and induces synapse regeneration after acoustic trauma. Elife. https://doi.org/10.7554/eLife.03564
Funding
This work was supported by a GSK Japan Research Grant 2019 and a JSPS KAKENHI grant (Grant Number 19K24052) (both to T.K).
Author information
Authors and Affiliations
Contributions
TK designed the experiments. TK, SM, MT and TY performed the experiments and analyzed the data. TK wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the Ethics Committee of the Kitasato University School of Medicine (2019–085).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kurioka, T., Mogi, S., Tanaka, M. et al. Activity-Dependent Neurodegeneration and Neuroplasticity of Auditory Neurons Following Conductive Hearing Loss in Adult Mice. Cell Mol Neurobiol 41, 31–42 (2021). https://doi.org/10.1007/s10571-020-00829-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-020-00829-y