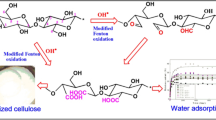
Abstract
The conventional TEMPO/NaBr/NaOCl system for oxidation of cellulose to prepare nanocellulose materials has some shortcomings in terms of controlling side reactions and clogging in washing/filtration process. A new TEMPO/CaBr2/Ca(OCl)2 system was then developed to oxidize a hardwood bleached kraft pulp (HBKP) in water at pH 10 (TEMPO = 2,2,6,6-tetramethylpiperidine-1-oxyl radical). An aqueous Ca(OH)2 solution was used to continuously control the reaction mixture at pH 10. After oxidation, the reaction mixture containing the oxidized products and chemicals was directly filtered on a 40-μm-mesh nylon filter and the water-insoluble oxidized products on the filter were washed with water without any clogging. The carboxy content increased to 1.5 mmol/g and the mass recovery ratio decreased to 87.7% as the amount of Ca(OCl)2 was increased to 10.0 mmol/g-HBKP. The oxidized products contained calcium ions but almost no chloride ions, indicating that they comprised almost pure –(COO)2Ca groups. The ready filtration and washing of the oxidized products was probably owing to the low degree of dissociation of the –(COO)2Ca groups in water. The X-ray powder diffraction (XRD) and solid-state carbon 13 nuclear magnetic resonance (13C-NMR) analyses revealed that the crystallinities and crystal widths of the original cellulose I structure were mostly retained in the oxidized products. However, size-exclusion chromatography and viscosity analyses revealed that substantial depolymerization occurred on the cellulose and oxidized cellulose molecules in the products, as in TEMPO/NaBr/NaOCl-oxidized products.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The 2,2,6,6-tetramethylpiperidine-1-oxyl radical (TEMPO)-catalyzed oxidation of polysaccharides in water has provided a new environmentally friendly and water-based chemistry (de Nooy et al. 1995; Isogai 2018, 2022). Oxidation using a TEMPO/NaBr/NaOCl system efficiently and position-selectively converts the primary C6–OH groups in polysaccharides to sodium C6-carboxylate groups. When the system is used to catalyze the oxidation of native crystalline cellulose, the products are convertible to anionic charge-containing cellulose microfibrils/nanonetworks/nanofibrils/nanocrystals, depending on the oxidation and successive mechanical fibrillation conditions (Isogai and Zhou 2019; Levanič et al. 2020; Liu et al. 2021; Isogai 2021).
When the TEMPO catalyst system is used to oxidize regenerated cellulose samples and other water-insoluble polysaccharides under suitable conditions, water-soluble products with almost homogeneous sodium C6-carboxylate groups are obtained (Isogai and Kato 1998; Chitbanyong et al. 2023). However, our detailed studies of the TEMPO/NaBr/NaOCl·5H2O-catalysed oxidation of hardwood and softwood bleached kraft pulps revealed that, depending on the oxidation conditions, substantial side reactions are unavoidable. Such side reactions include the depolymerization of cellulose and oxidized cellulose molecules in the oxidized products, and the formation of tailing structures of homogeneous sodium β-(1 → 4)-polyglucuronate chains in the water-insoluble oxidized products, both of which decrease the mass recovery ratio (Hou et al. 2023a, b, 2024). Furthermore, after TEMPO/NaBr/NaOCl-catalyzed oxidation, the pH of the reaction mixture comprising the oxidized products and other chemicals must be altered from ~ 10 to ~ 2.5 to convert the –COONa-type structures in the products to –COOH-type structures. This ensures trouble-free filtration and washing with water without clogging. The sodium carboxylate group-bearing TEMPO-oxidized products swell considerably in water owing to the high degree of dissociation of the –COONa groups (Hou et al. 2022; Hu et al. 2018). This causes clogging during washing by filtration.
Therefore, in the present study we investigated a wholly calcium-based TEMPO system in place of a sodium-based system for the oxidation of wood cellulose fibers to reduce the number of side reactions compared with the conventional TEMPO/NaBr/NaOCl oxidation system. We used a TEMPO/CaBr2/Ca(OCl)2 system to oxidize a representative sample of wood cellulose fibers, i.e., hardwood bleached kraft pulp (HBKP), in water, and maintained the pH of the reaction mixture at pH 10 during oxidation using a Ca(OH)2 solution. Subsequently, we determined the carboxy contents, yields, and degrees of polymerization of the water-insoluble oxidized products prepared with various amounts of Ca(OCl)2, and investigated their reaction kinetics and solid-state structures. Finally, we compared the results obtained using the Ca-based system with those obtained using a conventional TEMPO/NaBr/NaOCl system.
Experimental
Materials
Nippon Paper Industry (Tokyo, Japan) provided dry lap sheets of HBKP. The HBKP sheets were cut into small pieces and soaked in water for 3 days. The water-swollen pieces were defibrated using a blender and demineralized by soaking the HBKP fibers in water at pH ~ 2.5 for 30 min, and then thoroughly washing them in water by filtration. The wet HBKP fibers (solid content ~ 18%) were stored at 4 °C before use. Analytical grades of TEMPO, CaBr2, Ca(OCl)2, Ca(OH)2, Ca(BH4)2, LiCl, and chromatography-grade N,N-dimethylacetamide (DMAc) were purchased from FUJIFILM Wako Pure Chemicals Co. Ltd (Tokyo, Japan), and used as received, assuming 100% purities. A standard solution of 0.05 M NaOH and a 10% trimethylsilyl diazomethane (TMSD)/diethyl ether solution were commercial products (Wako Pure Chemicals). A 1.65 g/L Ca(OH)2 solution was prepared by dissolving Ca(OH)2 in water.
TEMPO/CaBr2/Ca(OCl)2 oxidation of HBKP
The wet HBKP (1 g of dry mass) was added to water (99 g), and TEMPO (0.1 mmol, 0.016 g) and CaBr2 (0.5 mmol, 0.097 g) were added to the HBKP slurry. The solid Ca(OCl)2 (1.25–10 mmol/g-HBKP) was added to the HBKP slurry while stirring. The pH of the HBKP slurry was kept at 10 by continuously adding the 1.65 g/L Ca(OH)2 solution using a pH stat (AUT-701, TOA DKK, Tokyo, Japan), and oxidation was continued at ~ 23 °C until there was no more consumption of Ca(OH)2. A few drops of ethanol were added to the mixture to decompose the residual Ca(OCl)2 to CaCl2. Then, Ca(BH4)2 (~ 0.01 g) was added to the mixture in the same container at pH 10 and ~ 23 °C. This post-reduction with Ca(BH4)2 continued for 3 h. The mixture was filtered on a 40-μm-mesh nylon filter, and the water-insoluble residue on the filter was repeatedly washed with water. Most of the oxidized product thus obtained was freeze-dried for determination of the mass recovery ratios and for structural analyses, and the rest was stored as a wet product at 4 °C.
Measurements of filtration time of the oxidized product/water slurries, and water-retention values of the oxidized products
After oxidation of HBKP (1 g of dry mass), the oxidized product/water slurry was filtered without drying on a 40 µm-nylon mesh filter to remove water-soluble degradation products and chemicals present in the slurry. The wet product was transferred to a beaker, and fresh water (250 mL) was added to the wet oxidized product. After sufficient stirring of the slurry with a magnetic stir bar, the filtration time of the slurry using a vacuum pump to remove water was measured (Fig. S1 in the Electronic Supplementary Material). The water retention values (WRVs) of the water-insoluble oxidized products in the wet state were measured using a 20 μm-nylon mesh filter according to ISO Standard 23714 (2014).
Analyses
Details of the following analytical methods and apparatus are mostly described in our previous paper (Hou et al. 2023a). The carboxy contents of the freeze-dried samples were determined by conductometric titration. The solid-state carbon 13 nuclear magnetic resonance (13C-NMR) spectra and X-ray diffraction (XRD) patterns of the oxidized products were obtained according to previously reported methods. The crystallinities of cellulose I and the crystal width of the (2 0 0) planes were calculated from the XRD patterns using the Segal’s and Scherrer’s equations, respectively (Segal et al. 1959; Alexander 1979). X-ray fluorescence (XRF) spectra of pressed pellet samples (~ 0.1 g each) of the oxidized products were obtained under vacuum to enable determination of the Ca and Cl contents using a Shimadzu EDX-8000 spectrometer at 500 μA and 50 kV for 100 s with or without a rhodium filter. The fiber morphologies were investigated using an optical microscope (Olympus BX53-P, Tokyo, Japan) with or without cross-polarizers.
The oxidized products with calcium carboxylate groups were converted to protonated carboxy groups by stirring the products in water at pH ~ 2.5 for 30 min, then thoroughly washing them with water by centrifugation and successive freeze-drying. The viscosity-average degrees of polymerization (DPv) of the oxidized products with protonated carboxy groups were measured with 0.5 M copper ethylenediamine oxide solution using a capillary viscometer. The DPv values of the oxidized products were calculated from their intrinsic viscosities ([η]) using the equation, [η] = 2.83 × DP0.67 (Hiraoki et al. 2015).
Methyl esterification of the protonated carboxy groups in the freeze-dried samples was performed with TMSD in methanol/DMAc according to a reported method (Hiraoki et al. 2015; Chitbanyong et al. 2023; Hou et al. 2024). The methyl-esterified product was repeatedly washed with ethanol and subsequently methanol by centrifugation. This once-methylated product was methylated again with TMSD under the same conditions. The twice-methylated sample was then washed with ethanol and subsequently t-butanol by centrifugation, and was obtained by freeze-drying. The original HBKP, and the oxidized and subsequently methyl-esterified samples were dissolved in 8% (w/w) LiCl/DMAc, which was diluted to 1% (w/v) LiCl/DMAc. The solutions were subjected to size-exclusion chromatography with multi-angle laser-light scattering and refractive index detection (SEC/MALLS/RI) using 1% (w/v) LiCl/DMAc as an eluent after filtration using a 0.45 μm-membrane to determine the molar mass parameters of the samples (Hiraoki et al. 2015; Ono et al. 2016; Hou et al. 2024). Details of the SEC/MALLS/RI system and operation conditions, and calculations of degrees of polymerization of the oxidized pulps from the molar mass parameters are described in the “SEC/MALLS/RI analysis section” of the Electronic Supplementary Materials. The mass-average and number-average degrees of polymerization (DPw and DPn, respectively) of the water-insoluble oxidized products were calculated from their mass-average and number-average molar masses (Mw and Mn, respectively), based on their degrees of oxidation (Hou et al. 2024). Details for calculations of the degrees of oxidation and polymerization from the carboxy contents, and Mw and Mn values of the oxidized products are described as the footnote of Table S1 in the Electronic Supplementary Materials. The molar mass parameters were measured three times for each sample.
Results and discussion
TEMPO/CaBr2/Ca(OCl)2 oxidation of HBKP prepared with various amounts of Ca(OCl)2
In accordance with our procedure for the oxidation of pulp in water at pH 10 using a TEMPO/NaBr/NaOCl system (Hou et al. 2023a), we oxidized HBKP using a wholly calcium-based system comprising TEMPO/CaBr2/Ca(OCl)2 with various amounts of Ca(OCl)2 and an aqueous Ca(OH)2 solution in water at pH 10. In the present study, we used HBKP as a representative of wood cellulose fibers because it contains ~ 83% high-molar-mass cellulose and ~ 17% low-molar-mass hemicelluloses (mainly xylan) (Hou et al. 2023a) without any chemical linkages between the two components (Ono et al. 2017, 2018; Ono and Isogai 2021).
The TEMPO/NaBr/NaOCl oxidation of HBKP produces water-insoluble products containing –COONa-type structures that swell markedly in water and clog filters when washed with water. In contrast, the TEMPO/CaBr2/Ca(OCl)2 system produced water-insoluble oxidized products that did not clog the filter when they were washed with water, probably owing to the low degree of dissociation of calcium carboxylate groups in water (Topp and Davies 1940; Heinze et al. 1993).
Figure 1 comprises optical microphotographs of the original HBKP and the TEMPO/CaBr2/Ca(OCl)2-oxidized products prepared with 5 or 10 mmol/g Ca(OCl)2, obtained with or without cross-polarizers. The HBKP fibers, which originally had an average length of ~ 1 mm, shortened as the amount of Ca(OCl)2 added during oxidation was increased. This indicates that some depolymerization occurred in the cellulose molecules of the HBKP during oxidation.
The carboxy contents and mass recovery ratios of the water-insoluble oxidized products are shown in Fig. 2a and Fig. S2 in the Supplementary Material. Here, the mass recovery ratios were calculated from the dried masses of the water-insoluble oxidized products and those of HBKP before oxidation. The yields of the oxidized products were calculated from the mass recovery ratios and carboxy contents of the water-insoluble oxidized products, considering the weight increase by introduction of Ca counterions into the carboxylate groups of the oxidized products. The results are shown in Table S1 in the Electronic Supplementary Materials, with the calculation methods of the yields, which should be ≤ 100%. The carboxy content increased up to 1.5 mmol/g and the mass recovery ratio decreased to 87.7% (corresponding to 83.4% yield, see Table S1) with increased amount of Ca(OCl)2 added to 10 mmol/g-HBKP in oxidation. As shown in Fig. 2b, the DPv value decreased from 1213 for the original HBKP to 193 with increased amount of Ca(OCl)2 added to 10 mmol/g-HBKP in oxidation, and it took ~ 21 h for complete oxidation. Thus, remarkable depolymerization occurred on the cellulose and oxidized cellulose molecules during TEMPO/CaBr2/Ca(OCl)2 oxidation.
Relationships between the amount of Ca(OCl)2 added during the TEMPO/CaBr2/Ca(OCl)2 oxidation of HBKP and: (a) the carboxy content and mass recovery ratio of the water-insoluble oxidized products; and (b) the viscosity average degree of polymerization (DPv) of the water-insoluble oxidized products and the reaction time required for oxidation
Some of the results in Fig. 2 are compared with those obtained for the TEMPO/NaBr/NaOCl-oxidized products reported previously (Hou et al. 2023a) together with the filtration times and WRVs, and are listed in Table 1. The mass recovery ratio, DPv, and reaction time for the TEMPO/CaBr/Ca(OCl)2-oxidized products were higher, higher, and shorter, respectively, than those of the TEMPO/NaBr/NaOCl-oxidized products at the same equivalent amount of the primary oxidants (i.e., Ca(OCl)2 and NaOCl, respectively) added in the two systems. However, the carboxy contents of the oxidized products prepared by the Ca-based system were lower than those prepared by the Na-based system at the same equivalent amount of the primary oxidants.
The slurries of the oxidized products containing calcium carboxylate groups exhibited short filtration times of 10 and 13 s, whereas those containing sodium carboxylate groups required remarkably long filtration times of > 1 h. In the latter cases, the water-swollen products partly clogged on the 40 μm-nylon mesh filter. The WRVs of the oxidized products supported the results of filtration times. The oxidized products prepared by the Ca-system exhibited WRVs of ~ 1.1 g/g, whereas those prepared by the Na-system showed 6 and 18 g/g; the oxidized products prepared by the Na-system were much more swollen in water than those prepared by the Ca-based system.
Chemical structures of the TEMPO/CaBr2/Ca(OCl)2-oxidized products
It is possible that the C6-carboxy groups in the water-insoluble TEMPO/CaBr2/Ca(OCl)2-oxidized products comprise –(COO)2Ca, –COOCaCl, and mixtures of these two structures (Uematsu et al. 2011). The freeze-dried products were then subjected to XRF analysis to determine the contents of calcium and chloride ions in the oxidized products. The XRF spectra of the oxidized products are shown in Fig. 3a. The oxidized products produced clear fluorescence peaks attributable to Ca, and the Ca peak intensity increased as the amount of added Ca(OCl)2 increased. In contrast, there were quite small or almost no peaks attributable to Cl in the spectra. The molar mass ratios of Ca:Cl in the oxidized products determined from the XRF spectra were in the range 1:0.003–0.015, demonstrating that almost all the carboxy groups in the oxidized products had the chemical structure –(COO)2Ca.
Figure 3b shows the peak intensities of CaKα of the oxidized products versus their carboxy contents, determined with or without a rhodium filter. There were strong linear relationships between the carboxy contents and the CaKα peak intensities of the oxidized products. Based on the –(COO)2Ca-type structures of the carboxy groups in the oxidized products, the degrees of oxidation were calculated from the carboxy contents in Fig. 2a, and the yields of the oxidized products were calculated from the mass recovery ratios in Fig. 2a and the degrees of oxidation. These results are shown in Table S1 in the Supplementary Material. The degree of oxidation of the products increased as the amount of added Ca(OCl)2 increased. Because the HBKP sample used in the present study contained ~ 83% cellulose and 17% hemicelluloses (Hou et al. 2023a), almost all the hemicelluloses originally present in the HBKP were probably degraded to water-soluble and low-molar-mass compounds, and were removed from the water-insoluble oxidized product by filtration when 10 mmol/g of Ca(OCl)2 was used.
Oxidation kinetics
The patterns of the time-dependent Ca(OH)2 consumption over the entire oxidation time are plotted in Fig. 4a, and those over a short reaction time of < 0.4 h are shown in Fig. 4b. The initial rate constants were similar between the oxidation reactions with various amounts of Ca(OCl)2 from Fig. 4a, whereas some differences were observed from Fig. 4b by magnification.
Based on the results shown in the previous section, the primary C6–OH groups in HBKP were oxidized to –(COO)2Ca groups by TEMPO/CaBr2/Ca(OCl)2 oxidation according to the equation provided in Fig. 5 below: two moles of Ca(OCl)2 and one mole of Ca(OH)2 are consumed to form one mole of –(COO)2Ca groups. There were large discrepancies between the theoretical values of the carboxy content at each amount of Ca(OCl)2 added in oxidation according to the equation in Fig. 5, and the actual values, which were clearly lower. Therefore, large amount of Ca(OCl)2 was consumed by side reactions other than the formation of calcium C6-carboxy groups in the water-insoluble fractions during oxidation. In contrast, the amount of Ca(OH)2 consumed at each amount of Ca(OCl)2 added in oxidation was approximately on the theoretical line. Thus, the Ca(OH)2 solution added to maintain the reaction mixture at pH 10 may have been consumed to neutralize some carboxy group-containing water-soluble degradation products formed from HBKP by oxidation with Ca(OCl)2 in water.
Solid-state structures of the TEMPO/CaBr2/Ca(OCl)2-oxidized products
The XRD patterns of the water-insoluble oxidized products are shown in Fig. 6a. The absolute diffraction intensities produced by the oxidized products decreased as the amount of Ca(OCl)2 added increased, although the pressed pellet samples subjected to XRD analysis had the same sample masses (~ 0.1 g each) and similar densities. The Segal’s crystallinities of cellulose I and the crystal widths calculated from the full widths at half height of the (2 0 0) diffraction peaks (French 2013) decreased slightly as the amount of Ca(OCl)2 increased (Fig. 6b). Therefore, the oxidation of HBKP by the TEMPO/CaBr2/Ca(OCl)2 system in water at pH 10 occurred almost position-selectively with regard to the C6–OH groups exposed on the crystalline cellulose microfibril surfaces, as in the case of the TEMPO/NaBr/NaOCl oxidation. The clear decrease in the XRD intensities of the oxidized products prepared with the larger amount of Ca(OCl)2 may have been caused by the calcium contents of the products, whereas the crystallinities did not decrease significantly.
Figure 7a shows the solid-state 13C-NMR spectra of the original HBKP and the water-insoluble oxidized products. The C = O signal area of the calcium carboxylate groups increased as the amount of Ca(OCl)2 increased. All the spectra contained peaks attributable to C6–OH groups at ~ 66 ppm, which corresponded to the tg conformation of the cellulose I crystal structure (Horii et al. 1982; Newman and Hemmingson 1994; Wickholm et al. 1998; Larsson and Westlund 2005; Yang et al. 2018; Sparrman et al. 2019). Although the NMR spectra were not obtained in the quantitative mode, the relative signal area ratios of C = O/C1 indicated an increase in the prevalence of calcium carboxy groups in the oxidized products as the amount of Ca(OCl)2 added during oxidation increased. The signal areas of the C4cry, C4non-cry, and C6-OH groups were obtained from the spectra by peak convolution using Gaussian/Lorentzian functions (Zhou et al. 2020; Hou et al. 2023a, 2024). The C6/C1 signal area ratio decreased and the C = O/C1 signal ratio of the oxidized products increased as the amount of Ca(OCl)2 increased (Fig. 7b). When the signal area ratios of C6/C1 and C = O/C1 were plotted against the carboxy contents of the oxidized products, good linear relationships were obtained (Fig. S3 in the Supplementary Material).
The relative signal area ratios of C4cry/C1 and C4cry/C4 indicate changes in the cellulose I crystallinity of the oxidized products. The signal area ratio patterns were not the same, although the C4cry/C1 and C4cry/C4 signal area ratios patterns were similar following the addition of Ca(OCl)2. The C4cry/C1 and C4cry/C4 signal area ratios decreased in the oxidized product prepared with 1.25 mmol/g Ca(OCl)2 compared with those of the original HBKP. However, the C4cry/C1 and C4cry/C4 signal area ratios changed little with the amount of Ca(OCl)2 added during oxidation, indicating that the oxidation of the C6–OH groups in the HBKP proceeded on the crystalline cellulose microfibril surfaces and not significantly inside the crystalline microfibrils.
Molar mass parameters of the TEMPO/CaBr2/Ca(OCl)2-oxidized products
The molar mass parameters of the water-insoluble oxidized products were analyzed by SEC/MALLS/RI after position-selective methyl esterification of the carboxy groups with TMSD in methanol/DMAc. The yields of the methyl-esterified products were more than 95% (Fig. S4 in the Supplementary Material), and the yield losses may have been caused by repeated handling and purification. Complete methyl esterification of the carboxy groups in the oxidized products was achieved by the method described in the Experimental section, and all the oxidized products were visibly dissolved in 8% (w/w) LiCl/DMAc after stirring for 1 day (Fig S5 in the Supplementary Material).
Figure 8a shows the SEC-elution patterns and corresponding molar mass plots of the oxidized/methyl-esterified products and the original HBKP, dissolved in 1% (w/v) LiCl/DMAc. When the SEC-elution patterns were detected by scattered laser light, which is sensitive to the presence of small amounts of aggregates not completely dissolved in a solvent, each sample produced a simple single peak (Fig. S6 in the Supplementary Material). This indicated that no insoluble aggregates were present in the solution and all the cellulose and oxidized/methyl-esterified molecules were completely dissolved at their individual molecular levels in the 1% (w/v) LiCl/DMAc. As shown in Fig. 8a, the molar mass plots linearly decreased with increasing SEC elution volume, demonstrating that all the molecules in the samples were adequately separated, depending on their sizes, by the SEC column. Furthermore, the molar mass plots of the samples were on the same line, showing that all the cellulose and oxidized/methyl-esterified cellulose molecules exhibited similar molar masses at each SEC-elution volume in the 1% (w/v) LiCl/DMAc.
(a) SEC-elution patterns and corresponding molar mass plots of the water-insoluble TEMPO/CaBr2/Ca(OCl)2-oxidized products, and (b) relationships between the amount of Ca(OCl)2 added during the oxidation of HBKP and mass-average, number-average, and viscosity-average degrees of polymerization (DPw, DPn, and DPv, respectively) and the DPw/DPn ratio of the oxidized products
The original HBKP showed a typical bimodal SEC-elution pattern, detected by RI, owing to the high-molar-mass cellulose and low-molar-mass hemicellulose fractions. The peak top position attributable to the high-molar-mass cellulose and oxidized cellulose fraction shifted to the higher SEC-elution volume or the lower molar mass direction of the oxidized products prepared with higher amounts of Ca(OCl)2. The peak attributable to the low-molar-mass hemicellulose fraction decreased as the amount of Ca(OCl)2 increased, and there was no clear difference between the high-molar-mass and low-molar-mass peaks produced by the oxidized products prepared with Ca(OCl)2 of > 5.0 mmol/g.
The mass-average and number-average degrees of polymerization (DPw and DPn, respectively) of the oxidized products were calculated from their mass-average and number-average molar masses, respectively, based on their degrees of oxidation (Hou et al. 2024) (Fig. 8b). The DPw/DPn values (or polydispersity values) and DPv values shown in Fig. 2b are also shown in Fig. 8b. The DP values clearly decreased as the amount of Ca(OCl)2 increased; substantial depolymerization could not be avoided by the TEMPO/CaBr2/Ca(OCl)2 system. Although there was a clear difference between the DPw and DPv values of the original HBKP, all the water-insoluble oxidized products prepared with the same amount of Ca(OCl)2 exhibited similar DPw and DPv values. This indicated the validity of the Mark–Houwink–Sakurada equation used to calculate the DPv values of the oxidized products obtained by the viscosity method (Hiraoki et al. 2015). The DPw/DPn values of the oxidized products were decreased by depolymerization.
The conformation plots and the double logarithmic plots of molar mass and root-mean-square radius of the oxidized products are shown in Fig. S7 in the Supplementary Material. All the samples produced linear plots with similar slopes of 0.51–0.60, indicating that all the cellulose and oxidized/methyl-esterified cellulose molecules had similar random-coil conformations in the 1% (w/v) LiCl/DMAc over their entire molar mass ranges.
Conclusions
In the present study, we developed a new TEMPO/CaBr2/Ca(OCl)2 system to oxidize a hardwood bleached kraft pulp in water at pH 10, and added various amounts of an aqueous Ca(OH)2 solution to continuously control the reaction mixture at pH 10. The oxidized products contained –(COO)2Ca-type structures. When the reaction mixtures containing the oxidized products and other chemicals were filtered on a nylon mesh and washed with water, the oxidized products did not cause any clogging. The carboxy content increased to 1.5 mmol/g and the mass recovery ratio decreased to 87.7% following oxidation when 10.0 mmol/g of Ca(OCl)2 was added. However, substantial amounts of Ca(OCl)2 were consumed by side reactions other than the formation of carboxy groups in the water-insoluble products, even during the TEMPO/CaBr2/Ca(OCl)2 oxidation of HBKP. XRD and solid-state 13C-NMR analyses of the oxidized products showed that the crystallinities and crystal widths of the original cellulose I of HBKP were mostly retained in the oxidized products. The SEC/MALLS/RI analysis and viscosity method revealed that considerable depolymerization occurred on the cellulose and oxidized cellulose molecules in the products during oxidation.
References
Alexander LE (1979) X-ray diffraction methods in polymer science. Robert E (Ed), Kreiger Publishing, Humington, New York, pp 423–424
Chitbanyong K, Hou G, Shibata I, Takeuchi M, Kimura S, Isogai A (2023) Polyglucuronic acids prepared from α-(1→3)-glucan by TEMPO-catalytic oxidation. Carbohydr Polym 330:121813. https://doi.org/10.1016/j.carbpol.2024.121813
De Nooy AEJ, Besemer AC, van Bekkum H (1995) Highly selective nitroxyl radical-mediated oxidation of primary alcohol groups in water-soluble glucans. Carbohydr Res 269:89–98. https://doi.org/10.1016/0008-6215(94)00343-E
French A (2013) Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 21:885–896. https://doi.org/10.1007/s10570-013-0030-4
Heinze T, Helbig K, Klemm D (1993) Investigation of metal ion adsorption of carboxymethyl cellulose gel beads. Acta Polym 44:108–109. https://doi.org/10.1002/actp.1993.010440210
Hiraoki R, Ono Y, Saito T (2015) Isogai A (2015) Molecular mass and molecular-mass distribution of TEMPO-oxidized celluloses and TEMPO-oxidize cellulose nanofibrils. Biomacromol 16:675–681. https://doi.org/10.1021/bm501857c
Horii F, Hirai A, Kitamaru R (1982) Solid-state high-resolution 13C-NMR studies of regenerated cellulose samples with different crystallinities. Polym Bull 8:163–170. https://doi.org/10.1007/BF00263023
Hou G, Li G, Chen H, Fang Z (2022) Rapid preparation of highly transparent paper with high built-in haze by an ion exchange approach. Chem Eng J 439:135776. https://doi.org/10.1016/j.cej.2022.135776
Hou G, Chitbanyong K, Takeuchi M, Shibata I, Isogai A (2023a) Comprehensive study of preparation of carboxy group-containing cellulose fibers from dry lap kraft pulps by catalytic oxidation with solid NaOCl. ACS Sustain Chem Eng 11:14782–14792. https://doi.org/10.1021/acssuschemeng.3c04750
Hou G, Chitbanyong K, Takeuchi M, Shibata I, Isogai A (2023b) Correction to “Comprehensive study of preparation of carboxy group-containing cellulose fibers from dry-lap kraft pulps by catalytic oxidation with solid NaOCl.” ACS Sustain Chem Eng 11:14782–14792. https://doi.org/10.1021/acssuschemeng.4c00215
Hou G, Chsitbanyong K, Takeuchi M, Shibata I, Isogai A (2024) Structural analyses of supernatant fractions in TEMPO-oxidized pulp/water reaction mixtures separated by centrifugation and dialysis. Carbohydr Polym 336:122103. https://doi.org/10.1016/j.carbpol.2024.122103
Hu W, Chen G, Liu Y, Liu Y, Li B, Fang Z (2018) Transparent and hazy all-cellulose composite films with superior mechanical properties. ACS Sustain Chem Eng 6:6974–6980. https://doi.org/10.1021/acssuschemeng.8b00814
Isogai A (2018) Review: Development of completely dispersed cellulose nanofibers. Proc Jpn Acad Ser B. 94:161–79. https://doi.org/10.2183/pjab.94.012
Isogai A (2021) Emerging nanocellulose technologies: Recent developments. Adv Mater 33:2000630. https://doi.org/10.1002/adma.202000630
Isogai A (2022) TEMPO-catalyzed oxidation of polysaccharides. Polym J 54:387402. https://doi.org/10.1038/s41428-021-00580-1
Isogai A, Kato Y (1998) Preparation of polyuronic acid from cellulose by TEMPO-mediated oxidation. Cellulose 5:153–164. https://doi.org/10.1023/A:1009208603673
Isogai A, Zhou Y (2019) Diverse nanocelluloses prepared from TEMPO-oxidized wood cellulose fibers: Nanonetworks, nanofibers, and nanocrystals. Curr Opin Solid St M 23:101–106. https://doi.org/10.1016/j.cossms.2019.01.001
Larsson PT, Westlund PO (2005) Line shapes in CP/MAS 13C NMR spectra of cellulose I. Spectrochim Acta Part A 62:539–546. https://doi.org/10.1016/j.saa.2005.01.021
Levanič J, Šenk V, Nadrah PP, Poljanšek I, Oven P, Happala A (2020) Analyzing TEMPO-oxidized cellulose fiber morphology: new insights into optimization of the oxidation process and nanocellulose dispersion quality. ACS Sustain Chem Eng 8:17752–17762. https://doi.org/10.1021/acssuschemeng.0c05989
Liu S, Low ZZ, Xie Z, Wang H (2021) TEMPO-oxidized cellulose nanofibers: a renewable nanomaterial for environmental and energy applications. Adv Mater Technol 6:2001180. https://doi.org/10.1002/admt.202001180
Newman RH, Hemmingson J (1994) Carbon-13 NMR distinction between categories of molecular order and disorder in cellulose. Cellulose 2:95–110. https://doi.org/10.1007/BF00816383
Ono Y, Isogai A (2021) Analysis of celluloses, plant holocelluloses, and wood pulps by size-exclusion chromatography/multi-angle laser-light scattering. Carbohydr Polym 251:117045. https://doi.org/10.1016/j.carbpol.2020.117045
Ono Y, Tanaka R, Funahashi R, Takeuchi M, Saito T, Isogai A (2016) SEC–MALLS analysis of ethylenediamine pretreated native celluloses in LiCl/N, N-dimethylacetamide: softwood kraft pulp and highly crystalline bacterial, tunicate, and algal celluloses. Cellulose 23:1639–1647. https://doi.org/10.1007/s10570-016-0948-4
Ono Y, Fujisawa S, Saito T, Isogai A (2017) Branched structures of softwood celluloses Proof based on size-exclusion chromatography and multi-angle laser-light scattering. ACS Symp Ser 1251:151–169. https://doi.org/10.1021/bk-2017-1251.ch008
Ono Y, Funahashi T, Saito T, Isogai A (2018) Stability of branched structures of softwood cellulose, investigated by SEC/MALLS/RI/UV and sugar composition analyses. Cellulose 25:2667–2679. https://doi.org/10.1007/s10570-018-1713-7
Segal L, Creely JJ, Martin A Jr, Conrad C (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text Res J 29:786–794. https://doi.org/10.1177/004051755902901003
Sparrman T, Svenningsson L, Sahlin-Sjovold K, Nordstierna L, Westman G, Bernin DA (2019) Revised solid-state NMR method to assess the crystallinity of cellulose. Cellulose 26:8993–9003. https://doi.org/10.1007/s10570-019-02718-0
Topp NE, Davies CW (1940) The extent of dissociation of salts in water. Part IX, Calcium and barium salts of dicarboxylic acids. J Chem Soc (resumed) 1940:87–93. https://doi.org/10.1039/JR9400000087
Uematsu T, Matsui Y, Kakiuchi S, Isogai A (2011) cellulose wet wiper sheets prepared with cationic polymer and carboxymethyl cellulose using a papermaking technique. Cellulose 18:1129–1138. https://doi.org/10.1007/s10570-011-9536-9
Wickholm K, Larsson PT, Iversen T (1998) Assignment of noncrystalline forms in cellulose I by CP/MAS 13C NMR spectroscopy. Carbohydr Res 312:123–129. https://doi.org/10.1016/S0008-6215(98)00236-5
Yang H, Wang T, Oehme D, Petridis L, Hong M, Kubicki JD (2018) Structural factors affecting 13C NMR chemical shifts of cellulose: a computational study. Cellulose 25:23–36. https://doi.org/10.1007/s10570-017-1549-6
Zhou Y, Ono Y, Takeuchi M, Isogai A (2020) Changes to the contour length, molecular chain length, and solid-state structures of nanocellulose resulting from sonication in water. Biomacromol 21:2346–2355. https://doi.org/10.1021/acs.biomac.0c00281
Acknowledgements
We thank Edanz for editing a draft of this manuscript.
Funding
Open Access funding provided by The University of Tokyo. GH thanks the China Scholarship Council (CSC) for financial support. KC is a recipient of a Japan Monbu-Kagakusho (MEXT) Fellowship for Foreign Ph.D. Students. This study was supported in part by the New Energy and Industrial Technology Development Organization (NEDO), Japan.
Author information
Authors and Affiliations
Contributions
GH performed most of the experiments and data analyses, and wrote the original draft. AI supervised the project and proposed the concept. GH, KC, IS, MT, and AI checked the obtained data. All authors read and approved the final manuscript for publication.
Corresponding author
Ethics declarations
Ethical approval
The present study did not comprise any experiments involving human participants or animals.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that may have influenced the work reported in this paper. The manuscript was approved for publication by all the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hou, G., Chitbanyong, K., Takeuchi, M. et al. TEMPO/CaBr2/Ca(OCl)2 oxidation of hardwood bleached kraft pulp in water at pH 10 with aqueous Ca(OH)2 solution. Cellulose (2024). https://doi.org/10.1007/s10570-024-06019-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10570-024-06019-z