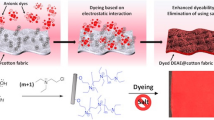
Abstract
The modification of cotton fabrics with water-soluble cationic copolymers for salt-free dyeing applications with reactive dyes is explored in the present work. To this end, a family of bifunctional water-soluble cationic copolymers was designed through the chemical modification of poly(4-vinyl benzyl chloride), PVBC, with triethylamine, TEAM. These copolymers were applied onto cotton fabrics in aqueous alkaline solutions at moderate temperature (60 °C). The successful cotton modification was verified through the characterization of the modified samples with Raman and XPS spectroscopy. Moreover, a polymer coverage of the fabric of the order of 0.1% (w/w) was estimated following the polymer exhaustion through UV–Vis spectroscopy of the aqueous solutions used for the modification. The modification kinetics were followed through the evolution of the Raman spectra of the modified fabrics, the polymer exhaustion of the aqueous modification solutions and, indirectly, the dye exhaustion of the dyebaths using a reactive dye, namely Novacron Ruby S-3B. All techniques claim that fabric modification is practically accomplished within the first 1–2 h. The double functionality of the copolymers was explored through the XPS study of the modified fabrics, revealing that both electrostatic binding and covalent bonding of the cationic copolymers with the anionic surface of the fabric are expected to take place. As an important consequence, the polymer-modified fabrics are strongly dyed under eco-friendlier conditions, namely in salt-free alkaline solutions at room temperature, in contrast to the barely dyed unmodified fabric.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cotton is one of the predominant cellulosic raw materials in the textile industry because of its advantageous properties, such as high hydrophilicity, biodegradability, good breathability, sense of comfort (Yetisen et al. 2016; Patil and Netravali 2019; Zhang et al. 2019). One of the most important challenges for the textile industry is the eco-friendlier dyeing of cotton fabrics. Currently, reactive dyes, namely dyes able to form covalent bonds with cellulose under alkaline conditions, are mostly used for cotton dyeing applications due to the wide range of bright hue, outstanding wash-fastness properties and ease of application (Acharya et al. 2014; Siddiqua et al. 2017). Reactive dyes are usually anionic and dyeing is performed in saline solutions of a high concentration to overcome the repulsive forces between the negative charges (under alkaline conditions) of cotton surface and the ionic species of dyes and increase dye exhaustion in the dyebath. However, this procedure has a detrimental effect in environment through the discharge of a highly colored and saline dyebath (Bhargava et al. 2019; Mu et al. 2019). Consequently, it is of great environmental and practical interest to find alternative “greener” methodologies for dyeing cotton fabrics with reactive dyes, avoiding the use of electrolytes (such as Na2SO4 or NaCl), as well as more sustainable processes in terms of energy consumption, avoiding application of high temperatures during dyeing processes.
An effective approach to avoid electrostatic repulsions between cellulose and reactive dyes is chemical modification of cotton fabrics by cationic compounds (Bashar and Khan 2013; Correia et al. 2020). This strategy leads to charge inversion of cotton fabrics’ surface, bypassing the utilization of electrolytes. The chemical modifiers may be low molecular weight compounds or polymer compounds. The most widely used low molecular weight cationic agent is 3-chloro-2-hydroxypropyl trimethylammonium chloride, CHPTAC (Acharya et al. 2014; Montazer et al. 2007; Fu et al. 2013; Arivithamani and Giri Dev 2016, 2017, 2018; Zhai et al. 2022; Pruś et al. 2022; Wang et al. 2022), while other agents such as 2,3-epoxypropyl-trimethylammonium chloride (Tarbuk et al. 2014), 2,3-epoxypropylaminopyridine (Huang et al. 2022) etc., have been also investigated. Nevertheless, such compounds have significant drawbacks, such as uneven dyeing and high process costs due to their low substantivity to cellulose and poor thermal stability (Fang et al. 2017), in addition to toxicity or unpleasant odor. Polymer compounds with cationic (Dehabadi et al. 2012; Ma et al. 2015; Wang et al. 2018; Yang et al. 2019; Song et al. 2020) or amine groups (Janhom et al. 2006; Zhang et al. 2007), are also studied and proposed for cationization of cotton. In most cases, covalent attachment of polymer onto the cellulose surface is assured through cellulose-initiated via UV (Shin et al. 1999), redox-initiated (Srikulkit and Larpsuriyakul 2002;) Ma et al. 2015; Ma et al. 2020) plasma (Ramamoorthy et al. 2016) or radiation (Goel et al. 2009) induced free radical polymerization or Atom Transfer Radical Polymerization (Dong et al. 2020) of adequate cationic monomers. Very recently, chemical reaction of cellulose with reactive units, like glycidyl methacrylate (GMA) of adequately designed functional cationic copolymers containing both cationic and reactive units, has been reported as an effective method to graft cationic polymers onto the cellulose surface (Niu et al. 2020).
4-vinyl benzyl chloride (VBC) is a versatile functional unit for the design of functional polymeric materials, since chlorine is a readily leaving group after the nucleophilic attack of tertiary amines or hydroxyl groups among others. In fact, we have taken advantage of the reaction of tertiary amines with adequate copolymers of VBC for the preparation of quaternized copolymers (Kougia et al. 2015) and polyampholytes (Koromilas et al. 2014), aiming at the preparation of crosslinked biocidal membranes and coatings (Druvari et al. 2016, 2021; Tsagdi et al. 2020). In the present work, we develop an alternative family of partially quaternized poly(4-vinyl benzyl chloride), PVBC, derivatives through reaction with triethylamine, TEAM, to obtain a series of cationic copolymers P(VBC-co-VBCTEAMx) of 4-vinyl benzyl chloride, VBC, with 4-vinyl benzyl triethylammonium chloride, VBTEAM (Scheme 1). Taking advantage of the reactivity of benzyl chloride towards cellulose for the preparation of benzyl cellulose derivatives (Gomberg and Buchler 1921, Rohleder and Heinze 2010; Li et al. 2011; Abe et al. 2017), the remaining VBC units of the P(VBC-co-VBCTEAMx) copolymers offers the possibility of covalent attachment, in addition to electrostatic binding of the polycations with the negatively charged cotton surface under alkaline conditions. Aiming at more sustainable processes, water-soluble cationic copolymers have been targeted, making possible the application of an eco-friendly process both for the cationic modification of the cotton surface in an aqueous environment and the effective salt-free dyeing of cationized cotton with reactive dyes. In addition, elaborated surface characterization techniques (see XPS) are applied to explore the bifunctional character of the copolymers.
Experimental
Materials
The monomer 4-vinylbenzyl chloride (VBC), the initiator azobisisobutyronitrile (AIBN), triethylamine (TEAM), deuterium oxide (D2O), deuterated chloroform (CDCl3) and NaCl were purchased from Sigma-Aldrich (Steinheim, Germany). The solvents N,N-dimethylformamide (DMF), dimethyl sulfoxide (DMSO), ethyl acetate, methanol, acetone and diethyl ether were purchased from Fischer (Pittsburgh, Pennsylvania, WI, USA). C.I reactive red mixture-89157-03-9; 718,619–88-6 (Novacron Ruby S-3B) was purchased from Huntsman International LLC. Cotton fabrics were kindly granted from COLORA S.A. Deionized water was obtained by means of an SG apparatus water purification unit.
Synthesis of polymers
Synthesis of PVBC
The homopolymer poly (4-vinylbenzyl chloride) (PVBC) was synthesized via free radical polymerization of 4-vinylbenzyl chloride (VBC) monomer using AIBN as the initiator in DMF at 80 °C. Furthermore, a 250 mL round bottom flask, equipped with a magnetic stirrer and a reflux condenser, was degassed (flamed under vacuum) and filled with argon. Next, 14.00 mL VBC (99.34 mmol) and 75 mL DMF were added. Then, the initiator AIBN was added 0.0816 g (0.497 mmol, 0.5% over the total monomer concentration). The system was degassed, flushed with argon again and heated at 80 °C for 2 days. The reaction mixture was concentrated in the rotary evaporator. The product was received by precipitation in methanol, filtration, washing with methanol and drying under vacuum at 60 °C overnight. Moreover, the molecular weight of the homopolymer was calculated, Mw: 43,000 g/mol, through gel permeation chromatography (GPC) in chloroform.
Synthesis of P(VBC-co-VBCTEAMx)
The precursor homopolymer PVBC was further modified with triethylamine (TEAM) as follows. In a 250 mL round bottom flask, equipped with a magnetic stirrer, 12.00 g of homopolymer PVBC (78.63 mmol) was first dissolved in 120 mL of DMF and then 5.48 mL TEAM (36.31 mmol) was added to the solution. The system was left at room temperature for 3 days. The reaction mixture was concentrated in the rotary evaporator. The product was received by precipitation in acetone, filtered, washed with acetone and dried under vacuum at 60 °C overnight. The molar percentage (x) of the VBCTEAM units in the copolymer P(VBC-co-VBCTEAMx) was determined through 1H NMR characterization in D2O.
Synthesis of VBCTEAM
The monomer 4-vinylbenzyl chloride (VBC) was modified with triethylamine (TEAM) as follows. In a round bottom flask 100 mL, equipped with a magnetic stirrer and a reflux condenser, was degassed (flamed under vacuum) and filled with argon. Next, 10.00 mL VBC (70.96 mmol) and 50 mL DMF were added. Then, 12.86 mL TEAM (92.25 mmol) was added to the solution. The system was degassed, flushed with argon again and was left at room temperature for 3 days. The product was received by precipitation in diethyl ether, filtered, washed with diethyl ether and dried under vacuum at 40 °C overnight.
Synthesis of PVBCTEAM
The homopolymer PVBCTEAM was synthesized via free radical polymerization of VBCTEAM monomer using AIBN as the initiator in DMSO at 80 °C. A 100 mL round bottom flask, equipped with a magnetic stirrer and a reflux condenser, was degassed (flamed under vacuum) and filled with argon. After, 5.00 g VBCTEAM (19.70 mmol) and 40 mL DMSO were added. Next, the initiator AIBN was added 0.0162 g (0.0985 mmol, 0.5% over the total monomer concentration). The system was degassed, flushed with argon again and was heated at 80 °C for 2 days. The product was received by precipitation in ethyl acetate, filtered, washed with ethyl acetate and dried under vacuum at 60 °C overnight.
Modification of cotton fabrics
As a standard modification protocol, the water-soluble polymers P(VBC-co-PVBCTEAMx) and PVBCTEAM were dissolved in deionized water at a 0.1% (w/v) concentration containing sodium hydroxide (10 g/L). Then, cotton substrates were immersed in the polymer solution and left for 3 h at 60 °C under stirring. The ratio of cotton substrate weight (Wtex) to volume of polymer solution (Vpol) was Wtext: Vpol = 1: 40 (w/v). Afterwards, the modified cotton substrates were washed with deionized water 3 times at room temperature and dried at 80 °C overnight. For kinetics studies the polymer concentration was 0.05% (w/v) and the modification time varied from 0 up to 24 h, while a polymer concentration range 0.001%–1% (w/v) was investigated to evaluate polymer exhaustion.
For reasons of comparison, coating of cotton fabrics with the homopolymer PVBC was also performed. Cotton fabrics were immersed in a 1% (w/v) PVBC solution in DMF, containing sodium hydroxide (10 g/L), and left for 3 h at 60 °C under stirring. The ratio of fabric weight to volume of polymer solution was Wtext: Vpol = 1: 40 (w/v). Afterwards, the modified cotton substrates were washed 3 times with DMF and then 3 times with deionized water at room temperature and dried at 120 °C overnight.
Dyeing of cotton fabrics
For all dyeing studies, the reactive dye Novacron Ruby S-3B was dissolved in deionized water at 0.0025% w/v concentration containing sodium hydroxide (0.4 g/L) and sodium carbonate (5 g/L). Next, cotton fabrics were immersed in the dyeing bath and left for 3 h at room temperature under stirring. The ratio of fabric weight to volume of dyeing bath (Vdye) was Wtext: Vdye = 1: 495 (w/v). Finally, the dyed cotton fabrics were washed 3 times with deionized water at room temperature and dried at 80 °C overnight.
For reasons of comparison, unmodified cotton fabric was also dyed applying the same protocol, in the absence or in the presence of NaCl at a 0.9 M molar concentration.
Characterization techniques
Proton nuclear magnetic resonance (1H NMR)
The samples for 1H NMR characterization were prepared by dissolving the homopolymer PVBC in CDCl3, containing TMS internal standard and the copolymers P(VBC-co-VBCTEAMx), the homopolymer PVBCTEAM and the monomer VBCTEAM in D2O. The 1H NMR spectra were recorded on a Bruker AVANCE DPX 400 spectrometer (Bruker BioSpin GmbH, Magnet Division, Karlsruhe, Germany) at 400 MHz at 300 K.
Attenuated total reflection fourier transform infrared spectroscopy (ATR-FTIR)
The ATR-FTIR spectra of the polymers were recorded on a Bruker Alpha-II Diamond ATR Spectrometer of Bruker Optics GmbH (Ettlingen, Germany).
Gel permeation chromatography (GPC)
The molecular weight of the polymer PVBC was identified by a Polymer Lab chromatographer (Agilent Technologies, Santa Clara, CA, USA). The measurement was carried out at 25 °C by using a Marathon II HPLC pump, a Fasma 500 UV–Vis detector and two PLgel 5 µm mixed columns. The mobile phase was chloroform, polystyrene standards were used for calibration and the software Clarity v.3.0.07.662 was used for the spectra analysis.
UV–Vis spectroscopy
UV–Vis spectra were recorded using a Hitachi U-1800 spectrophotometer (Hitachi High-Technologies Europe GmbH, Mannheim, Germany). All UV–Vis measurements were performed in air using quartz cuvettes for the examination of solutions.
Raman scattering
Backscattering Raman spectra were collected using the T-64000 model of JobinYvon (Horiba group) excited with a Cobolt Fandango TM ISO laser, operating at 514.5 nm. The excitation beam was directed to the sample compartment of a properly modulated metallurgical microscope (Olympus BHSM-BH2). The microscope was used for the delivery of the excitation laser beam on the sample and the collection of the backscattered light through a beam splitter and the objective lens adapted to the aperture of the microscope. The focusing objective was a Long Working distance (10 mm) 100x/0.55 Olympus lens. The spectra were obtained using a 2 mW laser power on the specimen for a total integration time of 1000 s. A viewing screen connected to the microscope offered good sample positioning and beam focusing as well as direct surface inspection. The Raman scattered radiation was focused on the slit of a single monochromator after being passed through an appropriate edge filter for the elastic Rayleigh scattering rejection (LP02-514RU-25, Laser 2000, Cambridgeshire, UK). Dispersion and detection of the Raman photons were done by a 600-grooves/mm grating and a 2D CCD detector (operating at 140 K), respectively. The spectral slit width was approximately 7 cm−1. In order to avoid fluorescence background, the samples were photobleached for several minutes, before Raman spectra had been collected.
FT-Raman
Fourier Transform Raman. For bulk measurements (polymer powders) of the samples an FT-Raman system (EQUINOX 55 equipped with Bruker FRA-106/S component) was employed with excitation at 1064 nm (Nd-YAG laser). The spatial resolution and the penetration depth offered by the focusing optics were both in the order of ∼mm. Spectra were backscattered at a spectral resolution of 2 cm−1.
X-ray photoelectron spectroscopy (XPS)
X-ray photoelectron spectroscopy measurements were performed in UHV (Pressure ~ 5 × 10−10 mbar) system equipped with a hemispherical electron analyzer (SPECS, Phoibos 100-1D-DLD) and a non-monochromatized dual-anode Mg/Al X-ray gun. Survey and narrow scans of C 1s, O 1s, N 1s and Cl 2p spectra were recorded by using the X-ray source MgKα at 1253.6 eV photon energy. Two analyzer pass energies were used, 10 eV and 20 eV, giving a Full Width at Half Maximum (FWHM) of 0.85 eV and 1.0 eV for the Ag3d5/2 line, respectively. Spectra were accumulated and fitted using commercial software (SpecsLab Prodigy; Specs GmbH, Berlin). The XP core level peaks are deconvoluted with a mixed Gaussian – Lorentzian functions after a Shirley background subtraction.
Results and discussion
Synthesis and characterization of functional cationic copolymers
As previously mentioned, our main goal was the design of water-soluble functional cationic copolymers, derived from the homopolymer PVBC (Scheme 1). The homopolymer was prepared through free radical polymerization in an organic solvent, since it is water-insoluble. Its weight-average molecular weight and polydispersity index were determined through gel permeation chromatography and found 43,000 and 2.7, respectively. Partial quaternization of PVBC with triethylamine, TEAM, provided a series of cationic copolymers P(VBC-co-VBCTEAMx) (Scheme 1, Route 1) of VBC with VBCTEAM (namely VBC quaternized with TEAM), where x is the molar content of VBCTEAM units as found through 1H NMR characterization. From solubility tests it was verified that the copolymers with x > 30% maintain water-solubility. For comparison reasons, the cationic homopolymer PVBCTEAM was also synthesized. In order to avoid the presence of even marginal quantities of unreacted VBC units, quaternization of the monomer VBC with TEAM was now performed prior to the polymerization of the resulting cationic monomer VBCTEAM (Scheme 1, Route 2).
The copolymers were characterized through a variety of techniques. As example, the 1H NMR spectra of the homopolymers PVBC and PVBCTEAM are compared in Fig. 1 with the respective spectra of two P(VBC-co-VBCTEAMx) copolymers with x = 53 and 70%. In more detail, in the spectrum of the homopolymer PVBC in deuterated chloroform, the peaks attributed to the aromatic ring (b and c) are observed in the 6.3–7.3 ppm region, while the peak attributed to the -CH2Cl group (a) is observed at 4.6 ppm and the peaks attributed to the backbone (d) are observed in the 1.2–2 ppm region. In the spectra of the homopolymer PVBCTEAM and the two P(VBC-co-VBCTEAMx) copolymers in D2O, the peak of –CH2- group of TEAM (e) appears at 3 ppm, while the strong peak, attributed to the CH3- groups of TEAM (d) is superimposed on the peaks attributed to the backbone in the 1–2 ppm region. From the ratio of the integral of this region (d, f) over that of the aromatic ring (b and c), the actual composition x of the copolymers could be determined. The results, summarized in Table 1, are in a rather good agreement with the feed composition of the polymer quaternization reaction.
The characteristic vibrational modes of VBC and VBCTEAM can be identified in the spectra of P(VBC-co-VBCTEAMx), shown in Fig. 2. The contribution of each component becomes evident if the spectra of PVBC (x = 0) and PVBCTEAM (x = 100) are compared. A detailed list of Raman and FTIR bands for these two homopolymers is reported in Table S1. The distinctive bands of the -CH2Cl chemical species dominate both FTIR and Raman spectra for the case of PVBC. More specifically, the strong bands at 3006 cm−1 and 2958 cm−1 in the Raman spectra and their weaker counterparts in the FTIR spectra are attributed to the CH2 stretching vibrations of these molecular species (Socrates 2004). Deformation, wagging, twisting and rocking vibrations of CH2 can be also resolved most probably at ~ 1445, 1265, 1180 and 745 cm−1. The contribution of the first two in both Raman and FTIR spectra is considerable while the intensity of the rocking vibrations is weaker. The most characteristic band is the C–Cl stretching vibration (at ~ 675 cm−1) which is very strong in the FTIR spectra. Although its contribution is considerable in the Raman spectra it lies rather close to the PVBCTEAM band at 681 cm−1, which is of similar intensity. For the case of VBCTEAM, the vibrational bands of the aliphatic components of the tertiary amine are the most distinguishing. Hence, the CH3 asymmetric, symmetric stretching vibrations at ~ 2985 cm−1, 2950 cm−1 and the respective deformation vibrations at 1455 cm−1, 1400 cm−1 dominate the vibrational spectra. Additionally, the N+C4 asymmetric stretching vibration of the quaternary amine at ~ 930 cm−1 (Berg 1978), the symmetric one (Kabisch 1980) at ~ 780 cm−1 and the CH3 and CH2 rocking vibrations (1010 cm−1 and 785 cm−1), which, as expected, are stronger in the FTIR spectra, are the bands associated with PVBCTEAM. For symmetric quaternary species (e.g. tetramethylammonium) single symmetric and asymmetric vibrational modes are expected. In the case of VBCTEAM the lack of symmetry in quaternary ammonium may result in splitting of the asymmetric vibrational mode in two peaks most possibly the one at 903 cm−1 (Mendoza 2012). From the assignment of the above-mentioned peaks observed in the Raman and FTIR spectra of VBC and VBCTEAM polymers, it becomes obvious that for the copolymers P(VBC-co-VBCTEAMx), the relative intensity of the VBCTEAM and VBC vibrations are essentially related to the relative content of each unit along the polymer chain, namely x.
Apart from the Raman and FTIR spectroscopic data, the success of the polymer synthesis is furthermore justified by XPS spectroscopy. The XPS spectra collected from PVBC, PVBCTEAM and P(VBC-co-VBCTEAMx) copolymers in the spectral range of C1s, N1s and Cl2p orbitals are given in Fig. 3. The C1s spectral region may be fitted using three peaks: (i) one attributed to C atoms participating in the benzene ring, (ii) a second peak attributed to the C atoms of the backbone chain and (iii) a third one associated with C atoms attached to the quaternary N+ atom or/and –Cl atom (Moulder 1992; Topalovic et al. 2007; Wang et al. 2021). The fitting indicates that the contribution of the third peak is more pronounced for the case of PVBCTEAM, its intensity decreases by a factor of nearly 0.5 for the case of P(VBC-co-VBCTEAM53) and nearly vanishes in the PVBC spectrum. The intensity of the first peak is greater for the PVBC polymer a fact that is reasonable since for each PVBC monomer the proportion of the carbon participating in the benzene ring is 6 out of 9. It is interesting to notice that in the N1s only one peak is detected at 402 eV which is attributed to quaternary nitrogen (Topalovic et al. 2007; Wang et al. 2021). This peak is absent in PVBC. Finally, concerning the Cl2p spectral region, two doublets are recorded. The first at 197.0 eV is assigned to Cl− i.e. the quaternary N+ counter ion, while the second at 201.1 eV is assigned to neutral Cl of PVBC (Topalovic et al. 2007; Wang et al. 2021). A weak band at 201.1 eV in the spectrum of PVBCTEAM indicates that there exists a small proportion of non-reacted monomers during the synthesis of the homopolymer. By following a typical fitting procedure of the XPS spectra, the elemental composition of all studied homo- and co-polymers may be derived and is summarized in Table 1.
Modification of cotton fabrics
As a standard protocol, the modification of cotton fabrics in aqueous solutions containing the desired polymer and 0.25 M NaOH for 3 h at 60 °C was explored.
In fact, after drying the modified textile, no significant weight change has been observed. In addition, no indication of the presence of cationic polymers on the cotton surface was observable through ATR-FTIR investigation. However, the presence of cationic polymers on treated textiles was evident in the respective Raman spectra. As seen in Fig. 4, the only spectral feature originating from the cationic polymer in the Raman spectra of modified textiles, not interfering with any of the cellulose vibrational modes, is the band at ~ 1610 cm−1, attributed to the para-substituted phenyl ring of the polymer units. The intensity of this band is rather low with respect to the strong cellulose vibrational bands, suggesting that the polymer mass within the scattering volume is small relative to cellulose.
XPS investigation offers additional evidence on the presence of polymers on the surface of modified textiles. In order to evaluate the XPS spectra in modified textiles the reference spectra of the free-standing cotton fabric are required. It has to be stressed that there is a noticeable effect of X-Ray irradiation, which is observed by the intensity decrease in the XPS spectra collected from modified and non-modified fabrics. The procedure followed so that the most reliable data could be obtained for the materials under investigation is given in detail in the supplementary material (Section S2).
Comparison with existing detailed XPS spectra of treated textiles (Wang et al. 2021; Topalovic et al. 2007) indicates that the bleaching process of the textiles used in the current work may be considered mild. Hence in the respective XPS spectra significant contribution of C–C and C–H, as well as weak N peaks, are resolved (Fig. 5). Taking into account the general chemical formulae of the modifiers studied, the most characteristic XPS peaks are expected to be the quaternary N+ and Cl− bands associated with the VBCTEAM component, the Cl peak related to VBC component, while in the C spectral region the phenyl species of both VBC and VBCTEAM components are expected to contribute to the overall C band. Surface modification is demonstrated by the observation of peaks in the C–C and C=O as well as quaternary N+ and Cl− peaks at ~ 285, 402 and 197 eV respectively (Fig. 3). Similar peaks are observed in the XPS spectra of all modified fabrics using different types of homo-/co- polymers.
XPS spectra of neat (a–c) and modified with PVBC-co-VBCTEAM53 (d–f) cotton fabrics in three distinct spectral regions: C1s, N1s and Cl2p. Depicted fitting curves indicate different species; the most characteristic of which are the tertiary and the quaternized N+ as well as its counterion Cl−. Dashed curves in (d-f) correspond to the pristine fabric contribution
Apart from the direct observation of the presence of polymer modifiers on the surface of treated cotton fabrics through the aforementioned Raman and XPS studies, the modification was also indirectly observed through UV–Vis spectroscopy by the decrease of the polymer concentration in the aqueous treating solution. The latter is straightforward since all polymers exhibit a strong absorbance band at ~ 260 nm. As seen in Fig. 6, the absorbance of the polymer solution decreases considerably after treatment of the cotton fabrics. To quantify these observations, calibration curves associated with the 260 nm band were constructed for each polymer (Fig. S3). The polymer exhaustion from the modification bath and the % mass coverage of the textile with cationic polymer can thus be determined as
where c0 and c is the polymer concentration (mg/mL) before and after treatment of cotton fabrics, V (mL) is the solution volume and m(mg) is the mass of cotton fabric.
UV–Vis absorption spectra of the polymer solutions, used for cotton modification, before (solid lines) and after the immersion of cotton fabrics (dashed line) for a P(VBC-co-VBCTEAM53) and b PVBCTEAM. With the exception of the more dilute solution, all mother solutions were adequately diluted to a final concentration c0 = 0.3 mg/mL. The solutions after cotton treatment were respectively diluted
The results concerning Polymer Exhaustion and % Mass Coverage of cotton fabrics treated with aqueous PVBCTEAM or P(VBC-co-VBCTEAM53) solutions are given in Fig. 7 as a function of the initial polymer concentration, c0. As seen, polymer exhaustion decreases sharply with the polymer concentration, already from low c0. This indicates a low surface coverage of the cotton fabric with the cationic polymers. Indeed, Fig. 7 shows that the % Mass Coverage of the fabric increases strongly at low polymer concentration and soon, at an initial polymer concentration somewhat higher than 1 mg/mL, tends to a plateau value of the order of 1–1.5%. It is noteworthy that a similar behavior concerning polymer exhaustion and % Mass Coverage of the fabric is observed for both polymers, with the homopolymer PVBCTEAM to be slightly more effective than the copolymer P(VBC-co-VBCTEAM53). This behavior indicates that polymer binding onto the negative cotton surface is primarily an ion exchange process. Since both polymers are characterized by a high linear charge density, no significant variations are expected, as it is the case. To modulate the extent of polymer binding, copolymers containing a much lower cationic content should be explored. However, this is not possible with the P(VBC-co-VBCTEAMx) copolymers family since the products are not water-soluble for x < 0.3.
Modification kinetics of cotton fabrics
Τhe modification kinetics is easily monitored indirectly, following the characteristic absorption band at 260 nm in the respective UV–Vis spectra of the solutions used for the textile modification at various time intervals, covering a modification time of 24 h. Representative results for the modification with PVBCTEAM and P(VBC-co-VBCTEAM53) with an initial polymer concentration c0 = 0.5 mg/mL are shown in Fig. 8. For both polymers the absorbance decreases considerably in the first few hours, reaching a constant value for higher modification times.
These experiments were performed in individual aqueous polymer solutions for each treatment time. Characterization of the modified textiles through Raman spectroscopy offers direct monitoring of the modification kinetics. The spatial resolution offered by the technique indicated that the intensity of the characteristic stretching vibration of the para-substituted benzene ring at ~ 1610 cm−1 was significantly altered at different spots on the same sample. This suggests that the modification is not uniform at least down to the level determined by the technique’s spatial resolution (in the order of 1 μm2). In order to confront the issue, average spectra obtained from at least 20 different spots on each sample were extracted. Averaged Raman spectra obtained from samples treated with PVBCTEAM for various modification times are shown in Fig. 9. The spectra are normalized with respect to the 1481 cm−1 band attributed to cellulose, the intensity of which was found to be the less sensitive to the polarization of light and/or sample anisotropy (hence it was used as a reference band). It is seen that the intensity of the ~ 1610 cm−1 band increases significantly during the first hours of modification.
The dependence of the extent of modification on treatment time could also be indirectly evaluated through the color of the samples after dyeing at fixed conditions. The appearance of the cotton fabrics prepared for the kinetics study, after dyeing with the reactive dye Novacron Ruby S-3B under basic conditions at room temperature is shown in Fig. 10. It should be noted that salt-free dyeing was attempted in this study. As a result, the unmodified textile (modification time is 0 min) is barely dyed, as seen. In contrast, it is clear that the textile treatment applied in the present study enables the effective salt-free dyeing of cotton. In fact, the modified samples are more strongly dyed with the increase of modification time. This is evident mostly for the first stages of modification (up to a modification time of about 2 h).
The appearance of the cotton fabrics modified for different time intervals using the polymer P(VBC-co-VBCTEAM53), after dyeing with the reactive dye Novacron Ruby S-3B under alkaline conditions. co = 0.5 mg/mL. Standard dyeing conditions were applied. The unmodified fabric was also dyed in the presence of NaCl at a 0.9 M concentration
The remaining dye in the dyebath can be easily monitored through UV–Vis spectroscopy (Fig. 11). As seen, the absorption band of the dye, centered at ~ 545 nm, decreases strongly with the time of cotton modification with PVBCTEAM or PVBC-co-VBCTEAM53). The construction of the calibration curve of the dye under these conditions (Fig. S4) allows the quantification of the dye concentration in the dyebath and, thus, the determination of dye exhaustion, defined as
where C0 and C is the dye concentration (mg/mL), before and after dyeing of cotton, respectively.
Figure 12 summarizes the results for the modification kinetics of cotton samples, as determined through the three methodologies, i.e. polymer exhaustion, Raman spectroscopy (on modified cotton surface) and dye exhaustion. It is clear that the results of the three techniques are in a very good agreement, following the same kinetics curve: modification of cotton increases sharply within the first two hours of treatment, while a smooth increase is observed for higher modification times.
In agreement with the results reported in Fig. 7, the results presented in Fig. 12 evidence that the extent of modification is similar regardless of x, since the cationic content is high enough. This is also clearly expressed in the dyeing capability, as well as in the respective dye fixation values (Table S2), of cotton fabrics modified for 2 h with PVBCTEAM or P(VBC-co-VBCTEAMx) copolymers. As shown in Fig. 13, while the dye exhaustion of the unmodified sample in salt-free solutions is very low (indicating no dyeability), in the case of all modified fabrics the dye exhaustion in salt-free solutions is high, indicating effective dyeability, similar to that of unmodified samples in strongly saline solution. It should be noted that similar dye exhaustion trends, reflected also to large differences of the K/S values between modified and unmodified samples, were also observed with other reactive dyes or mixtures of reactive dyes, as well as acid dyes (Lada et al. 2023).
Dye exhaustion of fabrics modified with PVBCTEAM or P(VBC-co-VBCTEAMx) copolymers in salt-free alkaline solution, as compared to unmodified fabric in salt-free or strongly saline (0.9 M NaCl) alkaline solutions. The dye used was Novacron Ruby S-3B. Standard modification and dyeing conditions were applied
Chemistry of modification
Of particular interest is to understand the mechanism of bonding of P(VBC-co-VBCTEAMx) modifiers onto the cellulose fabrics. In principle, the polymers possess two possible active sites that can interact with the substrate (Scheme 2). The first is the VBC unit, which could covalently bind to the cellulose fabrics and the second is the VBCTEAM counterpart which may electrostatically interact with the oppositely charged sites of alkali-treated cellulose surface during the modification process. However, possible hydrolysis of VBC units should also be considered, when studying the interaction between the modifier and cotton surface (Mabey and Mill 1978). The low quantity of modifier on the cotton surface complicates further the study, since it prohibits the monitoring of C–Cl vibrational modes due to the strong interference with the corresponding cellulose vibrational bands. Confocal measurements were applied which however did not suppress the cellulose signal to a level that the modifier peaks were observed.
Careful XPS measurements, on the other hand, could offer valuable information on the physicochemical characterization of the cotton modification process, despite the disadvantage of samples’ degradation. The Cl2p spectral region of the P(VBC-co-VBCTEAM53) modifier is compared in Fig. 14 to that obtained from the respective modified fabric. As explained in Section “Synthesis and characterization of functional cationic copolymers” (Fig. 3, spectral region of Cl2p orbitals), the two doublets are assigned to Cl− of the VBCTEAM counterion (~ 197 eV) and the neutral Cl of the VBC unit (~ 200 eV) and are of approximately equal intensity indicating that the VBC/VBCTEAM analogy is basically the same. After the modification, the line profiles in the same spectral region are altered. More specifically, the neutral Cl bands are severely suppressed compared to the Cl− bands (Fig. 14). Since there is an overlap of the Cl− bands with the neutral Cl bands, a chemical protocol was followed that exchanges the counter Cl− anion with Br−. As verified, the Cl− band disappears now in the XPS spectra. On the other hand, a Br− band is clearly resolved in the respective spectral region. The elimination of the Cl− contribution reveals that the absence of the characteristic Cl band of the VBC segments is striking. In fact, a similar observation was also verified during the first stages of modification kinetics study, while VBC units were clearly observable in the modification solution, indicating that the origin of this complete disappearance of the characteristic Cl band of VBC is not just hydrolysis. In contrast, this is a strong indication that covalent attachment takes indeed place. Concerning electrostatic binding of the modifier with the surface of alkali-treated cellulose, the N+/Cl− atomic ratio should be monitored. This ratio may be used for the evaluation of the electrostatic binding between the VBCTEAM units and cellulose and it was found that it is considerably altered. However, the detection of peaks associated with the VBCTEAM counterions (either Cl− or Br−) in the modified fabrics suggests that this type of binding follows an ion exchange process.
This ion exchange process can be monitored by XPS spectroscopy on the samples, which were modified at selected time durations with the homopolymer PVBCTEAM. The N+/Cl− atomic ratio of the samples was calculated for all samples. As expected, the ratio is ~ 1 for the homopolymer PVBCTEAM, while its value increases with treatment time and soon reaches a plateau value of ~ 5–6 (Fig. 15). This indicates that the majority (more than 80%) of the compensating Cl− anions relative to the N+ cations is not present on the surface of the treated fabrics, as a consequence of electrostatic binding of the modifier’s polymer chains with the negative charges of the surface of alkali-treated cellulose. The detection of Cl− counterions, however, indicates that the surface of the fabric is positively charged, allowing the facile salt-free dyeing with the negatively charged reactive dyes, as evidenced in the previous section.
Conclusions
The eco-friendly modification of cotton fabrics with cationic copolymers for salt-free dyeing applications with reactive dyes is explored in the present work. The design of the cationic copolymers was based on the partial modification of PVBC with triethylamine, leading to water-soluble products containing both cationic units and unreacted VBC units. The designed copolymers are thus of a double functionality, namely they are both cationic (quaternized VBCTEAM units) and chemically reactive (the unreacted VBC units) towards cellulose.
The water-solubility of the copolymers allowed the application of an eco-friendly modification process of cotton fabrics, namely using aqueous solutions at moderate temperature. The quantity of the cationic copolymer on cotton fabric was low, so that it could not be evaluated by weight alteration or identified by FTIR spectroscopy. Raman and XPS spectroscopies were able to verify the modification of the cotton fabrics. Indirect monitoring of the modification kinetics was achieved by UV–Vis spectroscopy of the copolymer solution used for modification, while related evidence was directly obtained by Raman spectroscopy. The indirectly/directly extracted kinetic curves were comparable and revealed that modification takes place mostly within the first 1–2 h. Moreover, XPS experiments strongly supports the bifunctional character of the copolymers towards cellulose of cotton fabrics.
The dyeing capabilities of the modified fabrics were substantially enhanced, permitting effective dyeing with reactive dyes in alkaline salt-free solutions. For study reasons, standard conditions involving quite large cotton modification and dyeing times have been applied in the present work. In terms of economic and industrial application, these systems can be further optimized, as it concerns the modification and dyeing conditions (liquor to textile ratio, concentrations, time, temperature etc.) and possibly the combination of the two processes (modification and dyeing) as successive steps, avoiding intermediate drying steps. Such an optimization, leading to industrially applicable finishing and dyeing processes of cotton textiles, competitive to traditional cotton dyeing processes, will reveal the potential of the present polymeric materials as cationic modifiers of cotton for the design of alternative eco-friendlier dyeing processes.
References
Abe M, Sugimura K, Nishiyama Y, Nishio Y (2017) Rapid benzylation of cellulose in tetra-n-butylphosphonium hydroxide aqueous solution at room temperature. ACS Sustain Chem Eng 5:4505–4510. https://doi.org/10.1021/acssuschemeng.7b00492
Acharya S, Abidi N, Rajbhandari R, Meulewaeter F (2014) Chemical cationization of cotton fabric for improved dye uptake. Cellulose 21:4693–4706. https://doi.org/10.1007/s10570-014-0457-2
Arivithamani N, Giri Dev VR (2016) Salt-free reactive dyeing of cotton hosiery fabrics by exhaust application of cationic agent. Carbohydr Polym 152:1–11. https://doi.org/10.1016/j.carbpol.2016.06.087
Arivithamani N, Giri Dev VR (2017) Sustainable bulk scale cationization of cotton hosiery fabrics for salt-free reactive dyeing process. J Clean Prod 149:1188–1199. https://doi.org/10.1016/j.jclepro.2017.02.162
Arivithamani N, Giri Dev VR (2018) Characterization and comparison of salt-free reactive dyed cationized cotton hosiery fabrics with that of conventional dyed cotton fabrics. J Clean Prod 183:579–589. https://doi.org/10.1016/j.jclepro.2018.02.175
Bashar MM, Khan MA (2013) An Overview on Surface Modification of Cotton Fiber for Apparel Use. J Polym Environ 21:181–190. https://doi.org/10.1007/s10924-012-0476-8
Berg RW (1978) The vibrational spectrum of the normal and perdeuterated tetramethylammonium ion. Spectrochim Acta A Mol Biomol Spectrosc 34:655–659. https://doi.org/10.1016/0584-8539(78)80067-1
Bhargava R, Mehta S, Tripathi Y, Shivankar VS, Raichurkar PP (2019) Eco-friendly cationized dyeing of cellulosic fabric: a review. Colourage 31–38.
Correia J, Rainert KT, Oliveira FR, Valle RCSC, Valle JAB (2020) Cationization of cotton fiber: an integrated view of cationic agents, processes variables, properties, market and future prospects. Cellulose 27:8527–8550. https://doi.org/10.1007/s10570-020-03361-w
Dehabadi VA, Buschmann HJ, Gutmann JS (2012) Durable press finishing of cotton fabrics with polyamino carboxylic acids. Carbohydr Polym 89:558–563. https://doi.org/10.1016/j.carbpol.2012.03.047
Dong W, Zhou M, Li Y, Zhai S, Jin K, Fan Z, Zhao H, Zou W, Cai Z (2020) Low-salt dyeing of cotton fabric grafted with pH-responsive cationic polymer of polyelectrolyte 2-(N,N-dimethylamino)ethyl methacrylate Colloids Surf A Physicochem Eng Asp 594:124573. https://doi.org/10.1016/j.colsurfa.2020.124573
Druvari D, Koromilas ND, Lainioti GCh, Bokias G, Vasilopoulos G, Vantarakis A, Baras I, Dourala N, Kallitsis JK (2016) Polymeric quaternary ammonium-containing coatings with potential dual contact-based and release-based antimicrobial activity. ACS Appl Mater Interfaces 8:35593–35605. https://doi.org/10.1021/acsami.6b14463
Druvari D, Antonopoulou A, Lainioti GCh, Vlamis-Gardikas A, Bokias G, Kallitsis JK (2021) Preparation of antimicrobial coatings from cross-linked copolymers containing quaternary dodecyl-ammonium compounds. Int J Mol Sci 22:13236. https://doi.org/10.3390/ijms222413236
Fang K, Zhao H, Li J, Chen W, Cai Y, Hao L (2017) Salt-free dyeing of cotton fabrics modified with cationic copolymer nanospheres using an acid dye. Fibers Polym 18:400–406. https://doi.org/10.1007/s12221-017-6541-0
Fu S, Hinks D, Hauser P, Ankeny M (2013) High efficiency ultra-deep dyeing of cotton via mercerization and cationization. Cellulose 20:3101–3110. https://doi.org/10.1007/s10570-013-0081-6
Gomberg M, Buchler CC (1921) Benzyl ethers of carbohydrates. J Am Chem Soc 43:1904–1911
Goel NK, Rao MS, Kumar V, Bhardwaj YK, Chaudhari CV, Dubey KA, Sabharwal S (2009) Synthesis of antibacterial cotton fabric by radiation-induced grafting of [2-(Methacryloyloxy)ethyl]trimethylammonium chloride (MAETC) onto cotton Radiat Phys Chem 78:399–406. https://doi.org/10.1016/j.radphyschem.2009.03.011
Huang Z, Wu P, Yin Y, Zhou X, Fu L, Wang L, Chen S, Tang X (2022) Preparation of pyridine-modified cotton fibers for anionic dye treatment. React Funct Polym 172:105155. https://doi.org/10.1016/j.reactfunctpolym.2021.105155
Janhom S, Watanesk R, Watanesk S, Griffiths P, Arquero OA, Naksata W (2006) Comparative study of lac dye adsorption on cotton fibre surface modified by synthetic and natural polymers. Dyes Pigm 71:188–193. https://doi.org/10.1016/j.dyepig.2005.06.018
Kabisch G (1980) Raman spectra and crystal structure of polycrystalline tetramethylammonium salts. J Raman Spectrosc 9:279–285. https://doi.org/10.1002/jrs.1250090502
Koromilas ND, Lainioti GCh, Oikonomou EK, Bokias G, Kallitsis JK (2014) Synthesis and self-association in dilute aqueous solution of hydrophobically modified polycations and polyampholytes based on 4-vinylbenzyl chloride. Eur Polym J 54:39–51. https://doi.org/10.1016/j.eurpolymj.2014.02.009
Kougia E, Tselepi M, Vasilopoulos G, Lainioti GCh, Koromilas N, Druvari D, Bokias G, Vantarakis A, Kallitsis JK (2015) Evaluation of antimicrobial efficiency of new polymers comprised by covalently attached and/or electrostatically bound bacteriostatic species, based on quaternary ammonium compounds. Molecules 20:21313–21327. https://doi.org/10.3390/molecules201219768
Lada ZG, Mathioudakis GN, Pavlidou S, Goulas G, Anastasopoulos C, Bokias G, Andrikopoulos KS, Voyiatzis GA (2023) Comparative assessment of the dyeing process for pristine and modified cotton fabrics towards the reduction of the environmental fingerprint. Sustainability 15:3144. https://doi.org/10.3390/su15043144
Li MF, Sun SN, Xu F, Sun RC (2011) Cold NaOH/urea aqueous dissolved cellulose for benzylation: Synthesis and characterization. Eur Polym J 47:1817–1826. https://doi.org/10.1016/j.eurpolymj.2011.06.013
Ma W, Wang T, Li H, Zhang S (2015) Cotton fabric modification through ceric (IV) ion-initiated graft copolymerisation of 2- methacryloyloxyethyltrimethyl ammonium chloride to enhance the fixation of reactive dyes. Cellulose 22:4035–4047. https://doi.org/10.1007/s10570-015-0713-0
Ma W, Du S, Yan S, Yu X, Zhang Z, Zhang S (2020) Salt-free dyeing of modified cotton through graft polymerization with highly enhanced dye fixation and good strength properties. Polymers 12:462. https://doi.org/10.3390/polym12020462
Mabey W, Mill T (1978) Critical review of hydrolysis of organic compounds in water under environmental conditions. J Phys Chem Ref Data 7:383–415. https://doi.org/10.1063/1.555572
Mendoza AJ (2012) Thermal and electrochemical Degradation of anion exchange membranes. PhD Thesis, The Pennsylvania State University, Department of Materials Science and Engineering.
Montazer M, Malek R, Rahimi A (2007) Salt free reactive dyeing of cationized cotton. Fibers Polym 8:608–612. https://doi.org/10.1007/BF02875997
Moulder JF (1992) Handbook of X-ray photoelectron spectroscopy: a reference book of standard spectra for identification and interpretation of XPS Data Physical Electronics Division, Perkin-Elmer Corporation.
Mu B, Liu L, Li W, Yang Y (2019) High sorption of reactive dyes onto cotton controlled by chemical potential gradient for reduction of dyeing effluents. J Environ Manag 239:271–278. https://doi.org/10.1016/j.jenvman.2019.03.062
Niu T, Wang X, Wu C, Sun D, Zhang X, Chen Z, Fang L (2020) Chemical modification of cotton fabrics by a bifunctional cationic polymer for salt-free reactive dyeing. ACS Omega 5:15409–15416. https://doi.org/10.1021/acsomega.0c01530
Patil NV, Netravali AN (2019) Cyclodextrin-based “Green” wrinkle-free finishing of cotton fabrics. Ind Eng Chem Res 58:20496–20504. https://doi.org/10.1021/acs.iecr.9b04092
Pruś S, Kulpiński P, Zgondek EM, Wojciechowski K (2022) Eco-friendly dyeing of cationised cotton with reactive dyes: mechanism of bonding reactive dyes wit CHPTAC cationised cellulose. Cellulose 29:4167–4182. https://doi.org/10.1007/s10570-022-04521-w
Ramamoorthy A, Helmy HM, Rajbhandari R, Hauser PJ, El-Shafei A (2016) Plasma induced graft polymerization of cationic and fluorocarbon monomers into cotton: enhanced dyeability and photostability. Ind Eng Chem Res 55:8501–8508. https://doi.org/10.1021/acs.iecr.6b01069
Rohleder E, Heinze T (2010) Comparison of benzyl celluloses synthesized in aqueous NaOH and dimethyl sulfoxide/tetrabutylammonium fluoride. Macromol Symp 294:107–116
Shin H, Ueda M, Burkinshaw SM (1999) New methods of obtaining patterned dyeings on cellulosic fibres with anionic dyes: photo-modification using a methacryloyl quaternary ammonium compound. Dyes Pigm 41:11–17. https://doi.org/10.1016/S0143-7208(98)00039-4
Siddiqua UH, Ali S, Iqbal M, Hussain T (2017) Relationship between structure and dyeing properties of reactive dyes for cotton dyeing. J Mol Liq 241:839–844. https://doi.org/10.1016/j.molliq.2017.04.057
Socrates G (2004) Infrared and Raman characteristic group frequencies: tables and charts, 3rd edn. Wiley, New York
Song Y, Fang K, Bukhari MN, Ren Y, Zhang K, Tang Z (2020) Green efficient inkjet printing of cotton fabrics using reactive Dye@Copolymer nanospheres. ACS Appl Mater Interfaces 12:45281–45295. https://doi.org/10.1021/acsami.0c12899
Srikulkit K, Larpsuriyakul P (2002) Process of dyeability modification and bleaching of cotton in a single bath. Color Technol 118:79–84. https://doi.org/10.1111/j.1478-4408.2002.tb00142.x
Tarbuk A, Grancaric AM, Leskovac M (2014) Novel cotton cellulose by cationization during mercerization-part 2: the interface phenomena. Cellulose 21:2089–2099. https://doi.org/10.1007/s10570-014-0194-6
Topalovic T, Nierstrasz VA, Bautista L, Jocic D, Navarro A, Warmoeskerken MMCG (2007) XPS and contact angle study of cotton surface oxidation by catalytic bleaching. Colloids Surf A Physicochem Eng Asp 296:76–85. https://doi.org/10.1016/j.colsurfa.2006.09.026
Tsagdi A, Druvari D, Panagiotaras D, Avramidis P, Bekiari V, Kallitsis JK (2020) Polymeric coatings based on water-soluble trimethylammonium copolymers for antifouling applications. Molecules 25:1678. https://doi.org/10.3390/molecules25071678
Wang L, Hu C, Yan K (2018) A one-step inkjet printing technology with reactive dye ink and cationic compound ink for cotton fabrics. Carbohydr Polym 197:490–496. https://doi.org/10.1016/j.carbpol.2018.05.084
Wang L, Wen X, Zhang X, Yuan S, Xu Q, Fu F, Diao H, Liu X (2021) Durable antimicrobial cotton fabric fabricated by carboxymethyl chitosan and quaternary ammonium salts. Cellulose 28:5867–5879. https://doi.org/10.1007/s10570-021-03875-x
Wang L, Xie G, Mi X, Kang X, Zhu Q, Yu Z (2022) A single-step pad-steam cationisation and dyeing process for improving dyeing properties of cotton fabrics. Color Technol 138:509–521. https://doi.org/10.1111/cote.12608
Yang H, Fang K, Liu X, An F (2019) High-quality images inkjetted on different woven cotton fabrics cationized with P(St-BA-VBT) copolymer nanospheres. ACS Appl Mater Interfaces 11:29218–29230. https://doi.org/10.1021/acsami.9b07848
Yetisen AK, Qu H, Manbachi A, Butt H, Dokmeci MR, Hinestroza JP, Skorobogatiy M, Khademhosseini A, Yun SH (2016) Nanotechnology in textiles. ACS Nano 10:3042–3306. https://doi.org/10.1021/acsnano.5b08176
Zhai S, Li Y, Don W, Zhao H, Ma K, Zhang H, Wang H, Zhao Y, Li X, Cai Z (2022) Cationic cotton modified by 3-chloro-2-hydroxypropyl trimethyl ammonium chloride for salt-free dyeing with high levelling performance. Cellulose 29:633–646. https://doi.org/10.1007/s10570-021-04295-7
Zhang F, Chen Y, Lin H, Lu Y (2007) Synthesis of an aminoterminated hyperbranched polymer and its application in reactive dyeing on cotton as a salt-free dyeing auxiliary. Color Technol 123:351–357. https://doi.org/10.1111/j.1478-4408.2007.00108.x
Zhang Z, Ma Z, Leng Q, Wang Y (2019) Eco-friendly flame retardant coating deposited on cotton fabrics from bio-based chitosan, phytic acid and divalent metal ions. Int J Biol Macromol 140:303–310. https://doi.org/10.1016/j.ijbiomac.2019.08.049
Acknowledgments
This research has been co‐financed by the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call RESEARCH—CREATE—INNOVATE (project code: T1EDK- 03073), project: “Development of Surface Coating Processes in Cotton Yarns/Fabrics for Single Stage Dyeing with Reduced Environmental Impact” with acronym: ChromaSurf.
Funding
Open access funding provided by HEAL-Link Greece. European Union and Greek national funds.
Author information
Authors and Affiliations
Contributions
GB, GAV and JKK: conceived the idea. LT, KP, CA and ASB: performed the experiments. LS: performed the XPS studies, KSA, GB, GAV and JKK: discussed the results and wrote the manuscript. All authors have reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Consent for publication
All authors reviewed and approved final submission.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tsimpouki, L., Papapetros, K., Anastasopoulos, C. et al. Water-soluble quaternized copolymers as eco-friendly cationic modifiers of cotton fabrics for salt-free reactive dyeing applications. Cellulose 30, 6031–6050 (2023). https://doi.org/10.1007/s10570-023-05220-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-023-05220-w