Abstract
Synthetic routes for functionalization of cellulose membranes by polymer chains characterized by controlled structures are constantly developed to precisely adjust the properties of the prepared material while minimizing the impact on the membrane performance. The review presents a critical and integrative evaluation of prior research on atom transfer radical polymerization (ATRP) techniques, emphasizing methods carried out with diminished catalyst concentration that were used for grafting polymers from cellulose membranes. The paper introduces cellulose as a naturally-derived and efficient material for filtration membrane production focusing on the fundamentals of the cellulose structure, and the reasons, and advantages of using cellulose as a membrane-built substrate. It also covers fundamental mechanistic aspects of ATRP and introduces the basic principles of low ppm ATRP methods focusing on the latest reports. The works up to date concerning the functionalization of cellulose membranes by the “classic” ATRP concept, paying attention to the concentration of the complex used and synthetic methodology, as well as the final properties of the obtained materials are shown. Subsequent, low ppm ATRP techniques are discussed against the background of the “classic” approach in synthesizing bioactive surfaces and functional biomaterials based on the structure of cellulose membranes, with emphasis on the advantages of methods with diminished catalyst level as a more cost-effective and thus more compatible to use in a commercial application. The present work is a concise and perspective review, which shows both the achievements to date and broad prospects for the development of this issue in the coming years.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
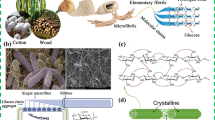
Membrane technology has emerged as a new promising and pervasive concept, e.g., for water purification due to its undoubted advantages over traditional technologies such as adsorption, distillation, and extraction. Considering substrates for membrane preparation, cellulose is an excellent candidate. It is the most abundant polymer material in nature. It possesses some promising properties as a material for membrane preparation, such as mechanical robustness, hydrophilicity, biocompatibility, and biodegradability (Kang et al. 2015; Tu et al. 2021). Cellulose is a major component of plant cell walls. It is an organic polysaccharide compound consisting of a linear chain of several hundred to many thousand β-1,4 glycosidic linkages D-glucose units (Kang et al. 2015). The membranes made of cellulose fibers are both chemically and mechanically stable with many applications in various industry and medicine branches (Liu et al. 2022b). However, due to its difficult dissolution, there are limited physiologically relevant or pharmaceutically clinical applications for this polymer. Moreover, considering the purpose of the membranes for purification, native cellulose membranes efficiently separate the components of the mixture against the molecular weight of the individual components (Kang et al. 2015).
Thus, the modification of cellulose membranes to improve their properties and extend their application is extensively studied. In this context, modification with polymers allows giving quite new properties for the membrane to separate components about their characteristics other than their molecular weight, e.g. charge and sensitivity to changes in pH (Bayramoglu and Arica 2021; Bhut and Husson 2009; Bhut et al. 2008; Chenette et al. 2012; Liu et al. 2009; Menkhaus et al. 2010; Pan et al. 2011; Porter et al. 2020; Qian et al. 2013; Singh et al. 2008b; Wang et al. 2015, 2014; Wei et al. 2011; Zeng et al. 2021; Zhang et al. 2011) or temperature (Pan et al. 2010a, b), as well as hydrophobic (Ye et al. 2020) or hydrophilic (Qiu et al. 2013) characteristics, etc. This allows for a precise separation with a wide range of applications in many industries. Polymers can also endow the characteristics of the membrane for use in tissue engineering or as an efficient wound dressing (Yang et al. 2016).
There is a critical need for controlled grafting of polymers from cellulose membranes to precisely adjust the properties of the prepared material. The properties of the modified cellulose membranes strongly depend on the grafting density (Yang et al. 2016), topology (Porter et al. 2022), structure, and molecular weight (Yang et al. 2016) of the attached polymer chains. It is also important to selectively modify only the outer or inner surface of the membrane (Carter et al. 2018; Sengupta and Wickramasinghe 2020), depending on the end-use of the product. It is also important to precisely control the polymerization while minimizing the effect on membrane performance.
Reversible deactivation radical polymerization (RDRP) techniques are currently one of the most influential solutions in the preparation of precisely-defined polymers with a predetermined structure and specialist purposes with a wide range of potential industrial applications (Matyjaszewski 2018; O'Brien and Ignaszak 2022; Truong et al. 2021; Zhou et al. 2022). It is compatible with the modification of a wide range of substrates, ranging from naturally-derived structures (Chmielarz 2018; Zaborniak et al. 2019b) going to flat surfaces (Chmielarz et al. 2017c; Flejszar et al. 2022; Kang et al. 2014, 2015) and nanoparticles (Alheety et al. 2022; Balis et al. 2020; Chmielarz et al. 2017d; Flejszar and Chmielarz 2019; Pu and Xu 2022), receiving functional hybrid nanomaterials, according to the “grafting from” approach. In this consideration, the atom transfer radical polymerization (ATRP) technique is especially privileged in the preparation of polymers with various architectures. The phenomenon of this method is associated with excellent control over molecular weight (MW), narrow molecular weight distribution (MWD) of received polymers (Chmielarz 2016; Qiao et al. 2022; Yin et al. 2021; Zaborniak et al. 2021b), ability to incorporate functionality in specific macromolecule places (Chmielarz 2017; Grześ et al. 2022; Matyjaszewski 2018), and possibilities for the optimization of a catalyst system (Fung and Coote 2021; Qiao et al. 2022; Zaborniak and Chmielarz 2022; Zhou et al. 2022). The above-mentioned features of ATRP make that technique an adequate tool for the modification of cellulose membranes. Up to now, the classical ATRP with high catalyst concertation (up to several hundred thousand ppm calculated with respect to the monomer concentration (Jiang et al. 2013)) has most often been used to modify cellulose membranes. The use of a high concentration of catalyst for polymerization from the surface requires long-term purification from the transition metal contamination, and the usability of such membranes is problematic for the separation of biomolecules. Moreover, modern polymer chemistry is heading towards the development of processes consistent with the "principles of green chemistry" to make syntheses more sustainable, therefore, the use of a high concentration of a metal catalyst is thriftless and generates a lot of waste for disposal (Anastas and Eghbali 2010; Dworakowska et al. 2022; Flejszar et al. 2021). The breakthrough in ATRP was the development of the techniques with the regeneration of catalyst complex in the form of an activator, significantly reducing the catalyst concentration to low ppm (Chmielarz and Sobkowiak 2017). Low catalyst loading methods offer more environmentally benign and industrially scalable reaction conditions compared to “classical” ATRP but also provide an option to prepare polymers with designed dispersity, defined by the catalyst content (Discekici et al. 2016, 2017, 2018; Lu et al. 2019; Matyjaszewski 2012a; Parkatzidis et al. 2020; Treat et al. 2014). Up to date, cellulose membranes were modified only a few times by low ppm ATRP techniques utilizing copper-based catalyst concentrations of 50–10,000 ppm and ascorbic acid to reduce the deactivated form of copper (II) halide catalytic complex to its active form (Bhut et al. 2012; Qiu et al. 2013; Worthley et al. 2011). Therefore, the main direction of research for cellulose membranes modification by controlled polymerization is the use of ATRP techniques with a reduced catalyst concentration. Development should focus mainly on concepts controlled by external factors, such as electric current (Chmielarz et al. 2017a; Luo et al. 2021; Zaborniak et al. 2020a; Zhao et al. 2021), ultrasound (Liu et al. 2022a; Mohapatra et al. 2017; Zaborniak and Chmielarz 2019a, b) and light (Corbin and Miyake 2022; Liu et al. 2020; Wei et al. 2022; Xu et al. 2022; Yan et al. 2020; Zaborniak et al. 2020b). Among externally controlled methods, light-triggered ATRP besides simple experimental setup, mild conditions with limited side reactions, easily accessible and tunable external stimuli source, and temporal control is the only externally controlled approach providing a metal-free catalyst system (Bian et al. 2019; Corbin and Miyake 2022; Discekici et al. 2016; Treat et al. 2014; Xu et al. 2021; Zhou et al. 2022, 2020). A full transition metal-free reaction setup is ensured by an organic photoredox catalytic cycle achieved by a single electron transfer from photoinitiator in the excited state (Theriot et al. 2016; Xu et al. 2022, 2021; Zaborniak and Chmielarz 2022; Zhou et al. 2020). The use of the light-mediated concept is the main goal to provide efficient membrane functionalization.
This concise review achieves an organization and statement of past work that significantly advances the membrane field in the context of functionalization by controlled radical polymerization techniques. The motivation for the proposed review paper is the high potential of the latest ATRP solutions for the modification of cellulose membranes, which are widely used in many industries. Therefore, it is necessary to prepare a literature review on the achievements to date in this field to precisely define the most promising prospects.
Cellulose–naturally-derived material for production of filtration membranes
Cellulose is a major component of plant cell walls. It is an organic polysaccharide compound consisting of a non-branched linear chain of several hundred to many thousand β-1,4 glycosidic linkages D-glucose units (Kang et al. 2015). Cellulose is the most abundant polymer material in nature and possesses some promising properties, such as mechanical robustness, hydrophilicity, biocompatibility, and biodegradability, therefore it has many applications for use e.g. in tissue engineering. It is one of the most promising substitutes for petroleum-derived synthetic polymers.
Pure cellulose can be found in nature in several allomorphs–cellulose I to IV, depending on the location of hydrogen bonds between and within strands of units. Native cellulose I exists in two distinct allomorphs–one chain triclinic structure Iα produced mainly by bacteria and algae, and two chains monoclinic unit cell Iβ, which is the main component of higher plants, and both allomorphs are packed in parallel chain arrangement. Cellulose I can be irreversibly transitioned to the form arranged antiparallel in a two-chain unit cell–cellulose II, a more stable crystalline form. This type of cellulose can be found in mercerized cotton and regenerated cellulose (RC). The treatment of native cellulose with anhydrous ethylamine or liquid ammonia leads to cellulose III structure, while cellulose IV is produced by heat treatment (Zugenmaier 2001).
The most common method for the preparation of cellulose membranes is the phase inversion technique (Khan et al. 2020)–the polymer is precipitated from a polymer solution film by a solvent which totally miscible with the solvent appropriate for the cellulose but does not dissolve the cellulose. Another one also includes the thermally induced phase separation (TIPS) method, namely, the homogenous solution of the cellulose is formed by dissolution in a diluent with a high boiling point, and then the temperature is reduced to induce a phase separation (Yu et al. 2017). Vapor-assisted nonsolvent induced phase separation (VNIPS) technique is also known, as being based on the dry–wet casting process, i.e. cellulose solution is exposed to a nonsolvent vapor (usual water), and then immersed in a coagulation bath (Wang et al. 2022a).
Cellulose membranes can be categorized as regenerated cellulose, cellulose membranes with hydroxyl groups blocked by acetate groups, or tertiary amine and benzyl groups. RC membranes are composed of cellobiose and have a high density of hydroxyl groups. Cellulosic acetate membranes have only 20–30% of unsubstituted hydroxyl groups (Vatanpour et al. 2022), while bulky chemical groups (tertiary amine (Zhang et al. 2019a) and benzyl moieties (Shokri et al. 2021)) substitute a relatively small fraction (< 5%) of the hydroxyl groups. Considering the usefulness of the cellulose membranes the substitution of the hydroxyl groups improved its properties in medical application because it prevents the interaction between hydroxyl groups present within the membrane structure and complement cascade products via steric hindrance (Eduok et al. 2021). Another strategy for the development of a more useful cellulose membranes is the modification of the hydroxyl groups by polymers using various concepts, making the final product hydrophobic and/or giving them different and quite new properties (Thakur and Voicu 2016).
Fundamentals of flat surfaces modification: atom transfer radical polymerization as a promising tool for membrane functionalization
Reversible deactivation radical polymerization (RDRP) techniques are currently one of the most influential solutions in the preparation of precisely-defined polymers with a predetermined structure and specialist purposes with a wide range of potential industrial applications (Cao et al. 2022; Matyjaszewski 2018). It is compatible with the modification of a wide range of substrates, ranging from naturally-derived structures (Chmielarz 2018; Zaborniak et al. 2019b) going to flat surfaces (Chmielarz et al. 2017c; Kang et al. 2014, 2015) and nanoparticles (Balis et al. 2020; Chmielarz et al. 2017d; Flejszar and Chmielarz 2019), receiving functional hybrid nanomaterials, according to the “grafting from” approach. In this consideration, the atom transfer radical polymerization (ATRP) technique is especially privileged in the preparation of polymers with various architectures. The phenomenon of this method is associated with excellent control over molecular weight (MW), narrow dispersity (MWD) of received polymers (Chmielarz et al. 2017c; Williams et al. 2015), ability to incorporate functionality in specific macromolecule places (Chmielarz et al. 2017a), and possibilities for the optimization of a catalyst system. The above-mentioned features of ATRP make that technique an excellent tool for the modification of cellulose membranes (Scheme 1a).
The mechanism of ATRP is based on the use of a transition metal complex as a catalyst, which participates in redox reactions, thus controlling polymerization (Krys and Matyjaszewski 2017; Matyjaszewski 2012b) (Scheme 1b). Usually, alkyl halide or polymer chain terminated with a halogen (PnX) reacts with reduced catalytic complex (activator, CuIL). As a result, propagating radicals (P˙) and oxidized catalytic complex (deactivator, XCuIIL) are formed. Initially developed classic ATRP concept suffers from several major disadvantages. It is necessary to use a large amount of a catalyst, up to several hundred thousand ppm, thus it generates additional costs, due to the necessity to use additional stages for purification of the polymerization products. Moreover, the catalyst is introduced to the reaction mixture in its reduced form, which is sensitive to the action of oxygen from the air. Therefore, the preparation for the catalytic complex is demanding and time-consuming because it must take place under an inert gas atmosphere, and consequently, it is also necessary to use the freeze-pump-threw cycle to remove the oxygen completely from the reaction setup. One disadvantage was solved by the use of a reducing agent to generate the active form of the catalytic complex in activators generated by electron transfer (AGET) ATRP approach. Therefore, the catalyst (reduced form) susceptible to the action of oxygen from the air is introduced to the reaction mixture in the form of a deactivator, thus every step of preparing the catalytic complex and introducing it to the flask may take place in the air atmosphere (Szczepaniak et al. 2021).
Modern polymer chemistry is heading towards the development of processes consistent with the "principles of green chemistry" to make syntheses more sustainable, therefore the use of a high concentration of a metal catalyst is thriftless and generates a lot of waste for disposal. The breakthrough in ATRP was the development of the techniques with the regeneration of catalyst complex in the form of an activator, significantly reducing the catalyst concentration to low ppm (up to 1 ppm (Chmielarz and Sobkowiak 2017)). Low catalyst loading methods offer more environmentally benign and industrially scalable reaction conditions compared to “classical” ATRP but also provide an option to prepare polymers with designed dispersity, defined by the catalyst content (Discekici et al. 2018, 2016, 2017; Lu et al. 2019; Matyjaszewski 2012a; Parkatzidis et al. 2020; Treat et al. 2014). The new low ppm ATRP methods regenerate the activator by the use of a chemical reducing agent as a radical initiator in initiators for continuous activator regeneration (ICAR) ATRP (Konkolewicz et al. 2012; Luan et al. 2022), chemical compounds (Luo et al. 2022; Matyjaszewski et al. 2007; Min et al. 2007) and silver (Williams et al. 2015) in activators regenerated by electron transfer (ARGET) ATRP–used a few times for membrane modification (Bhut et al. 2012; Worthley et al. 2011), zerovalent metals in supplemental activator and reducing agent (SARA) ATRP (Chmielarz et al. 2015a; Konkolewicz et al. 2014, 2013), external stimuli as an electrical current in electrochemically mediated ATRP (eATRP) (Chmielarz 2017; Chmielarz et al. 2015b; De Bon et al. 2022; Han et al. 2022; Park et al. 2015; Zhao et al. 2022), light in metal-free (Soly et al. 2022; Su et al. 2022; Treat et al. 2014) or photochemically mediated ATRP (photo-ATRP) (Fu et al. 2021; Huang et al. 2017; Tasdelen et al. 2011), mechanical forces in mechanically induced ATRP (mechano-ATRP) (Liu et al. 2022a; Mohapatra et al. 2018; Wang et al. 2019), ultrasound in ultrasonication-induced atom transfer radical polymerization (sono-ATRP) (Wang et al. 2018; Zaborniak and Chmielarz 2019a) or ultrasonication-light mediated ATRP (Wang et al. 2022b). Low catalyst loading methods enable a significant reduction of the catalyst concentration, and the use of naturally-derived chemical compounds, or external stimuli as reducing agents, which allows the design of an economic reaction setup, simultaneously providing well-defined polymer structures with various architecture (Matyjaszewski 2012a; Pan et al. 2018).
Currently, the most intensively developing ATRP technique is light-controlled controlled polymerization in which the catalytic complex has been eliminated from the reaction system (Liu and Yi 2018; Razeghi et al. 2021; Soly et al. 2022; Xu et al. 2021). In this consideration light-triggered ATRP besides simple experimental setup, mild conditions with limited side reactions, easily accessible and tunable external stimuli source, and temporal control is the only externally controlled approach providing a metal-free catalyst system (Discekici et al. 2016; Treat et al. 2014). A full transition metal-free reaction setup is ensured by an organic photoredox catalytic cycle achieved by a single electron transfer from photoinitiator in the excited state. This approach usually uses organic photocatalysts such as pyrene and anthracene (Allushi et al. 2016), perylene and phenazines (Theriot et al. 2016), phenoxazines (Park et al. 2019), and phenothiazines (Discekici et al. 2016; Treat et al. 2014) derivatives for direct reduction of the alkyl halide initiator. It is achieved due to appropriate characteristics in the excited state e.g. stable triplet state and sufficient negative reduction potential, or reductive quenching pathway with an additional electron donor (Bian et al. 2018) to enable efficient reduction of the initiator. The recent achievements include the complete elimination of synthetic photocatalyst structures by their environmentally friendly counterparts, e.g. riboflavin (Zaborniak et al. 2020b, 2021a) or curcumin (Zaborniak and Chmielarz 2022). Externally controlled ATRP methods have never been used to modify membranes and are therefore of particular importance in future perspectives.
From the ATRP point of view, among various types of cellulose membranes the most interesting are regenerated cellulose membranes due to the high density of hydroxyl groups in their structures because when using the ATRP technique for precise grafting of polymers from this type of substrates, the "grafting from" concept should be applied (Balis et al. 2020; Chmielarz et al. 2017b; Macior et al. 2022; Zaborniak et al. 2019a, 2021b; Zhang et al. 2021). In this concept, polymerization is initiated directly from the initiator (alkyl halide) attached to the substrates by an esterification reaction. It provides the formation of well-ordered and dense layers composed of surface-attached macromolecules with extended conformation. These thin organic layers covalently grafted to various flat/porous surfaces enable the formation of a wide range of materials with specific use.
Cellulose membrane modification by high catalyst concentration ATRP concept
The classical ATRP has most often been used to modify cellulose membranes compared to the low ppm concepts. The ATRP of different vinyl monomers was initiated by cellulose modified with halogen initiators and catalyzed with copper salts in the presence of various amine-type ligands. The details for the modification of cellulose membranes via high catalyst surface-initiated classical ATRP are summarized in Table 1. Cellulose membranes were grafted with polymers with various characteristics using the high catalyst ATRP technique to improve or give membranes novel properties, e.g. formation of cation- (Bayramoglu and Arica 2021; Chenette et al. 2012; Liu et al. 2009; Porter et al. 2020; Qian et al. 2013; Wang et al. 2015, 2014; Zhang et al. 2011), anion-exchange (Bhut and Husson 2009; Bhut et al. 2008; Menkhaus et al. 2010; Pan et al. 2011; Qian et al. 2013; Singh et al. 2008b; Wei et al. 2011; Zeng et al. 2021) or multifunctional (Feng et al. 2014; Liu et al. 2014; Porter et al. 2022; Yang et al. 2016; Ye et al. 2020; Zhao et al. 2010) adsorption membrane for proteins (Bhut et al. 2008; Chenette et al. 2012; Himstedt et al. 2013; Liu et al. 2010; Qian et al. 2013; Singh et al. 2008b; Wang et al. 2015, 2014; Zhao et al. 2010) or enzymes (Feng et al. 2014; Zeng et al. 2021) absorption. Cation, anion or zwitterion surfaces are created by polymerization of monomers with functional group, e.g.–polyelectrolytes (Chen and Lee 2021), from the flat surface.
Cation exchange cellulose-based surfaces were prepared by polymerization of acrylic acid (AA) from the membrane (Menkhaus et al. 2010; Pan et al. 2010b, 2011; Singh et al. 2008b). Poly(acrylic acid) (PAA) contains many carboxyl groups, which under pKa (~ 4.5) are protonated, while in alkaline conditions are deprotonated (Hu et al. 2015; Wang et al. 2009). Hence, PAA forms a negatively charged anion capable of adsorbing cations in high pH aqueous solutions, while under low pH conditions positively charged molecules desorb. Regenerated cellulose membrane was firstly grafted with PAA using up to ca. 626,616 ppm (catalyst to monomer ratio) of copper(I) chloride (CuICl) catalyst. The modification decreased the permeability of the membrane, however, in comparison to commercially available ion-exchange membranes, higher static and dynamic binding capacity for lysozyme as a model protein was obtained (Singh et al. 2008b). Due to the ability of negatively charged acrylic acid residues to intermolecular cyclization in aqueous ATRP, in effect leading to loss of chain-end functionalities and ultimately a cessation of the polymerization (Lorandi et al. 2020), the syntheses were carried out in an alkaline environment with the addition of a salt–sodium chloride, forming an ion pair with acid residues (Menkhaus et al. 2010; Pan et al. 2010b, 2011; Singh et al. 2008b). Among commercially available regenerated cellulose membranes, electrospun cellulose acetate membrane was modified by PAA (Menkhaus et al. 2010) with the same catalyst complex–CuICl/1,4,8,11-tetraazacyclotetradecane (Me4Cyclam), nevertheless a much lower catalyst concentration was used–ca. 7000 ppm. The polymer grafting imparted the membrane with 50-fold higher adsorption capacities of lysozyme compared to commercial membrane adsorption systems and over 12-fold higher than packed bed resins. Moreover, PAA-based membranes demonstrated tenfold faster adsorption kinetics than packed bed resins and the permeance was over 15-fold higher.
Another concept for incorporation of acrylic acid moieties on cellulose membrane is the surface-initiated polymerization of (meth)acrylates with tert-butyl side chains (Porter et al. 2022, 2020). Elimelech et al. (Porter et al. 2020) grafted poly(tert-butyl acrylate) (PtBA) chains from films and membranes, controlling the location of brush growth using a sealing wafer (Fig. 1b,c). Tert-butyl moieties were then hydrolyzed to PAA brushes to elaborate a novel concept for direct measurements of brush density, namely by binding silver ions to PAA brush layers grafted from dense cellulose films by carboxyl group deprotonation, followed by anions elution (Fig. 1a). As a controlled character of ATRP allows the incorporation of other polymer blocks to an existing chain due to the presence of halides at the chain ends, the surface of the membranes grafted with PtBA was further modified with poly(2 hydroxyethyl acrylate) (PHEA) to create a membrane with selective capabilities of brush layer (Fig. 1d–f).

Reproduced from Ref. (Porter et al. 2020) with permission. Copyright 2019 Elsevier B.V
a Hydrolysis of tert-butyl moieties from tBA attached to cellulose membrane to acrylic acid side chains, followed by determination of grafting density by silver-binding method; b Components of a sealing wafer to control brush growth location; c Photographs of the sealing wafer; d Characteristics of solutes involved in transport experiments for diblock copolymer brush-layered cellulose membranes, where LogP is an octanol/water partition coefficients; e Fluxes of solutes with varying LogP coefficients through bare and modified membranes i.e. membrane modified with bromide, homopolymer chains, and block copolymers; f Rejections of sodium chloride and lysozyme, and pure water permeability measured for unmodified cellulose membrane and a membrane grafted with PtBA.
Another monomer with easily hydrolyzed tert-butyl groups is tert-butyl methacrylate (tBMA). The monomer was used for the preparation of polyelectrolyte multilayer membranes (PEMs) by the sequential, layer-by-layer deposition of polyelectrolytes–poly(tert-butyl methacrylate) hydrolyzed to a negatively charged poly(methacrylic acid) (PMAA) block and poly(2-dimethyl)aminoethyl methacrylate (PDMAEMA) quaternized to a positively charged block, on porous supports (Fig. 2a) (Porter et al. 2022). The prepared PEMs exhibited favorable ion-ion selectivity and significant salt rejection at relatively high water flux (Fig. 2b, c). PDMAEMA is another type of polyelectrolyte–a weak polybase that contains tertiary amine groups.

Reproduced from Ref. (Porter et al. 2022) with permission. Copyright 2021 Elsevier B.V
a Synthesis of polyelectrolyte multilayer membranes (PEMs) by initial modification by ATRP initiator and crosslinker, followed by functionalization with poly(tert-butyl methacrylate) (PtBMA) and PDMAEMA, and conversion to charged blocks by hydrolysis of tert-butyl moieties of PtBMA to receive negatively charged poly(methacrylic acid) (PAA) and quaternization of amines in PDMAEMA receiving a positively charged poly(2-(methacryloyloxy)ethyl trimethylammonium iodide) (PMOTA) block; b Various salt rejection by bare and modified membranes; c The proposed hypothesis for diminished salt rejection with an increasing number of blocks.
The functional groups are positively charged at low pH, becoming a typical cationic polyelectrolyte, while at high pH the amino groups are deprotonated (Han et al. 2013). Husson et al. used relatively low catalyst loading in classical ATRP (ca. 550 ppm) for polymerization of 2-(dimethylamino)ethyl methacrylate (DMAEMA) from RC membranes creating high-capacity anion-exchange membranes for chromatographic bioseparation (Bhut and Husson 2009; Bhut et al. 2008). The prepared materials exhibited high static and dynamic binding capacity measured for bovine serum albumin (BSA) up to 140 mg/mL and 130 mg/mL, respectively (Bhut and Husson 2009). The measurements were performed at pH 7.4 when PDMAEMA is charged positively, and BSA carried a net negative charge. Then the ion exchange interaction is possible because it is most efficient between the isoelectric point (pI) of protein (pI of BSA is ca. 4.7–4.9) and acid dissociation constant (pKa) of the polymer–the pKa value for the tertiary amine group in DMAEMA side chains is up to 7.9 (Brilmayer et al. 2020). A significantly higher catalyst concentration (26,848 ppm) was used for the preparation of PDMAEMA-based cellulose membranes for laccase immobilization. The modified membrane was characterized by approximately 3.3 times higher immobilization amount compared to the initial RC membrane (Zeng et al. 2021). This type of material can be effectively used for recycling enzymes. Over a dozen of million ppm of catalyst was utilized for grafting of PDMAEMA from RC membrane to obtain a material capable of removing Cu2+ ions due to the high reactivity of the amino groups in the formation of strong complexes with metallic ions (Jiang et al. 2013).
The polymer successfully used for cellulose membrane modification by classical ATRP (2500–46,090 ppm of catalyst loading) is poly(glycidyl methacrylate) (PGMA). The polymer contains three-membered epoxide side chains in its structure, therefore it is susceptible to the nucleophilic ring-opening reactions of the pendent epoxide groups. Post-polymerization modification of PGMA allows for the incorporation of a variety of functionalities onto the reactive scaffold, thus membrane with a wide range of new properties can be obtained (Muzammil Ezzah et al. 2017). It is possible to receive anion-exchange membrane by derivatization of PGMA with diethylamine (Qian et al. 2013), and also materials with cation-exchange properties (Bayramoglu and Arica 2021; Wang et al. 2015, 2014) by ring-opening reaction with 4-mercaptobenzoic acid (Wang et al. 2015, 2014) or orthophosphoric acid and sulfuric acid (Fig. 3a) (Bayramoglu and Arica 2021). Both types of membranes were characterized by high pH-dependent static capacity evaluated for BSA (Qian et al. 2013) and immunoglobulin G (IgG) (Wang et al. 2015, 2014) protein. While cation-exchange films with phosphate and sulfate groups were successfully used for the removal and recovery of Chlorazole Black E (CBE) dye from aqueous solutions depending on adsorbent dosage, pH, ionic strength, initial concentration of dye, and contact time of the adsorbate and the adsorbent receiving the maximum adsorption capacities up to 165.7 and 268.6 mg/g for the phosphate- and sulfate-based membrane, respectively (Fig. 3b, c) (Bayramoglu and Arica 2021). Cellulose membrane with polyhydroxyl functional groups for efficient boron complexation was received by reaction of N-methylglucamine with epoxide rings (Wei et al. 2011).

Reproduced from Ref. (Bayramoglu and Arica 2021) with permission. Copyright 2021 Elsevier B.V
a Synthetic route for the preparation of polymer grafted cellulose membrane with phosphate and sulfate reactive groups in side chains; b Absorption capacity (q) of membranes functionalized with sulfate and phosphonate groups depending on the initial concentration of Chlorazole Black E dye; c The reusability of the cellulose-based adsorbers for adsorption–desorption cycle of Chlorazole Black E dye.
A further concept for the preparation of absorptive membranes with functional groups including e.g. –SO3H (or –SO3Na), –PO4H, and –NH2 through the ATRP approach is the polymerization of monomers belonging to the group of strong cation exchangers i.e. 3-sulfopropyl methacrylate potassium salt (SMP) (Chenette et al. 2012; Zhang et al. 2011), sodium 4-styrenesulfonate (NASS) (Qian et al. 2013), or zwitterion molecules i.e. sulfobetaines–N,N-dimethyl-N-(p-vinylbenzyl)-N-(3-sulfopropyl) ammonium (DMVSA) (Liu et al. 2009, 2010, 2014) and [2-(methacryloyloxy)-ethyl]-dimethyl-(3-sulfopropyl)ammonium hydroxide (SBMA) (Liu et al. 2010, 2014; Zhao et al. 2010), and phosphobetaine–2-methacryloyloxyethyl-phosphorylcholine (MPC) (Liu et al. 2010, 2014). In the majority of the presented papers, the cellulose membranes grafted with the above-mentioned polymers were prepared in an aqueous-alcoholic environment (Liu et al. 2009, 2010, 2014; Qian et al. 2013; Zhang et al. 2011; Zhao et al. 2010), however, using usually a high concentration of the catalyst, from about 4,000 (Chenette et al. 2012) to as much as 250,000 ppm (Zhang et al. 2011).
Strong cation-exchange membranes with sulfonate as the exchange groups were examined in terms of application for protein (lysozyme) capture (Chenette et al. 2012; Qian et al. 2013). The static and dynamic binding capacities measurements indicate the PNASS-based layers as more efficient for lysozyme binding, reaching a maximum capacity of 138.3 mg/mL and 92.9 mg/mL (Qian et al. 2013) vs. 79.0 mg/mL and 70.0 mg/mL–PSMP grafted CM (Chenette et al. 2012) for static and dynamic adsorption, respectively. Beside, PNASS modified membrane effectively adsorbed an optical indicator ligand for cadmium ions–5,10,15,20-tetrakis(1-methyl-4-pyridinio) porphyrin tetra(p-toluenesulfonate) (TMPyP) (Zhang et al. 2011). Therefore, it was found the polymer-modified cellulose-based material possessed performance for selective removal of cadmium ions, even in the presence of other cationic species such as K+, Na+, Ca2+, and Mg2+ ions existing together in the aqueous solutions. The multifunctional membrane enabled also rapid detection of cadmium ions with the naked eye without using other sophisticated instruments due to the quick color change (within 2 min) from yellow to green when it was exposed to cadmium ions even at relatively low ion concentrations.
Sulfobetaines–a special class of zwitterionic polyelectrolytes with ammonium cation and sulfonic anion located on the same monomer residue, were grafted from cellulose membranes (Fig. 4a) to receive stimuli-responsive porous membranes with varying permeation/separation properties (Liu et al. 2009, 2010, 2014; Zhao et al. 2010). The membrane modified with SBMA separated BSA from model impurities (polystyrene nanoparticles of different sizes) depending on the change of NaCl concentration in the solutions. The membrane allowed BSA to permeate regardless of the presence of NaCl, whereas the rejection rates of nanoparticles changed remarkably with the concentration of NaCl in the solutions (Zhao et al. 2010). Both sulfobetaines (Liu et al. 2009, 2010) and phosphobetaine (Liu et al. 2010) grafted from CM significantly improved the blood compatibility of the modified materials, namely, zwitterionic brushes endowed the functionalized CMs with improved resistance to nonspecific protein adsorption and platelet adhesion examined on fresh human platelet-poor plasma (PPP) made of fresh blood. Another essential parameter of blood-contacting biomaterials for biomedical applications is cytocompatibility. CM substrates modified with zwitterionic polymers exhibited improvement in cytocompatibility evaluated by 2D and 3D cell culture, namely, a direct culture of the cells (rat bone marrow stem cells) on CM-modified materials and in situ encapsulation of cells in crosslinked polymethacrylate hydrogels bearing the respective zwitterionic motifs, respectively (Liu et al. 2014). The results of cytocompatibility of zwitterion-based cellulose membranes were comparable to poly(ethylene glycol) (PEG)-based coatings (Fig. 4b–e).

Reproduced from Ref. (Liu et al. 2014) with permission. Copyright 2014 Royal Society of Chemistry
a Surface modification of cellulose membranes with zwitterionic and poly(ethylene glycol) methacrylate (PEGMA) brushes; b In situ morphology of the cells cultured on various cellulose membrane substrates after 24 h, with the original culture medium (left side), after gentle rinsing with fresh PBS (right side). Examination of c water contact angle, d total protein absorption after 90 min of incubation in fresh human PPP, and e viability of rat bone marrow stem cells (rMSCs) cultured with different CM substrates up to 3 days, for bare, brominated and polymer modified cellulose membranes.
Another material with potential application as an effective hemostasis dressing based on electrostatic interactions was developed by functionalization of a biocompatible cellulose membrane with choline phosphate, namely, the membrane was grafted with N-(11-azido-3,6,9-trioxaundecanyl) methacrylamide monomer in water/methanol environment, followed by functionalization with substrates bearing multiple choline phosphate groups via click chemistry (Yang et al. 2016). The mechanism of bioadhesion of human red blood cells is based on a universal adhesion reaction between phosphatidylcholine (PC)–a major component of biological membranes, and CM grafted with polymer brushes carrying a choline phosphate (CP) group per monomer. The adhesion of human blood cells was strongly dependent on CP density, thus the molecular weight of the tethered polymer chains, while grafting density affected the properties much less. The authors also evaluated a novel method for determining the brush density for these rough surfaces by atomic force microscopy (AFM) technique. The brush densities were estimated on the hydrated materials through calibration and interpretation of force-distance curves simultaneously applying the Alexander theory of brush structure to relate to each other the three essential parameters of the polymer brushes, namely the measured brush height with the chain density and the estimated polymer molecular weight.
An approach different from the preparation of cellulose membranes with ion-exchange coatings is grafting the CM surface with thermosensitive (Pan et al. 2010a, b) and simultaneously anti-fouling polymers (Liu et al. 2014; Singh et al. 2008a; Wandera et al. 2012, 2011). Molecules of this type are soluble in water, therefore the polymerizations were conducted in an aqueous or aqueous/alcoholic environment usually with content from about 12,000 ppm to over several hundred thousand ppm of a copper-based catalyst (Pan et al. 2010a; Singh et al. 2008a; Wandera et al. 2012, 2011). The best-known thermosensitive polymer is poly(N-isopropylacrylamide) (PNIPAM). The polymer aqueous solution undergoes transitions between the water-soluble state and the insoluble state above the lower critical solution temperature (LCST) of approximately 31 °C to 33 °C (Jain et al. 2015). The CM modified by PNIPAM exhibited thermally modulated permeability properties, namely, flux values increased as the temperature increased (Pan et al. 2010a). Another concept includes polymerization of biocompatible, non-toxic, and miscible with many solvents PEG-based macromolecules from CM surface. The modification allowed for receiving the materials with adjustable pore sizes evaluated based on dextran rejection experiments (Singh et al. 2008a). The controlled characteristics of the ATRP process enable the synthesis of block copolymers due to the presence of initiation sites at the end of the polymer chains, thus PNIPAM-b-PEGMA block copolymers were successfully grafted from cellulose membranes (Fig. 5a–c) (Wandera et al. 2012, 2011). The motivation for grafting this type of polymer blocks from a material with a cellulose structure was the nature of PEGMA to suppress attachment of foulants, while PNIPAM was selected for its thermoresponsive feature. As a result, materials with controlled fouling during filtration of oily water were obtained to provide a chemical-free strategy to reverse foulant accumulation (Fig. 5d, e). Other stimuli-responsive membranes based on thermo-sensibility were prepared by grafting poly N-vinylcaprolactam (PVCL) from microfiltration cellulose films (Himstedt et al. 2013). PVCL exhibits LCST around 32 ºC, which can be altered by changing the ionic strength of the buffer solution. Below LCST the chains swell while above this temperature separation occurs and the polymer adopts a collapsed and dehydrated conformation (Maeda et al. 2002). The prepared material was used for efficient absorption and recovery of BSA based on hydrophobic interaction chromatography (HIC) behavior.

Reproduced from Ref. (Wandera et al. 2011) with permission. Copyright 2011 Elsevier B.V. Flux versus filtration time for a bare and two modified membranes modified by d NIPAM for 0.5 and 2.0 h, followed by PEGMA for 3 h, and e by NIPAM for 1.0 h, and then PEGMA for 3.0 h. Reproduced from Ref. (Wandera et al. 2012) with permission. Copyright 2011 Elsevier B.V
a Modification of cellulose membrane by PNIPAM-b-PEGMA copolymer; AFM topographical dry layer images (1 μm × 1 μm) of b unmodified cellulose membrane, root mean square (RMS) roughness = 2.6 nm, and c CM grafted with PNIPAM-b-PEGMA copolymer, RMS roughness = 1.7 nm.
Two different mixed-mode hydrophobic/ion-exchange (HIC/IEC) and reversed-phase/ion-exchange (RPC/IEC) membrane adsorbers were prepared by modification of RC membrane with ATRP of styrene-thiolactone (St-Tla), followed by the amine-thiol-ene conjugation using N-butylamine/methacrylic acid and octylamine/methacrylic acid as the modifier. The mixed-mode membranes after optimization of how the solution pH, salt type, ionic strength, and solvent content affect the protein absorption demonstrated a similar statistic adsorption capacity, i.e. 73.5 μg/cm2 for IgG (pH = 8 and 0.5 mol/L NaCl in PB buffer), and 70 μg/cm2 for α-chymotrypsin (α-CTP) (pH = 6.5 in PB buffer) respectively (Ye et al. 2020).
Classic ATRP concept suffers from several major disadvantages. One disadvantage was solved by the use of a reducing agent to generate the active form of the catalytic complex in AGET ATRP approach. Therefore, the catalyst susceptible to the action of oxygen from the air is introduced in the form of a deactivator to the reaction mixture, thus the very step of preparing the catalytic complex and introducing it to the flask may take place in the air atmosphere. AGET ATRP was successfully used for the polymerization of SBMA (Xu et al. 2020), [2-(acryloyloxy)ethyl]trimethylammonium chloride (DAC) (Xu et al. 2020), 2-hydroxyethyl methacrylate (HEMA) (Carter et al. 2018), GMA (Sengupta and Wickramasinghe 2020) and DMAEMA (Wang et al. 2013) monomers from cellulose membrane surfaces (Table 2). The polymerizations were conducted in water/methanol environment, in the presence of ascorbic acid (AsAc) as a reducing agent in an equimolar amount or in molar insufficiency with respect to the catalyst, using a copper concentration from about 2000 to 90,000 ppm.
Grafting copolymer brushes consisting of a zwitterion–poly([2-(methacryloyloxy)-ethyl]-dimethyl-(3-sulfopropyl)ammonium hydroxide) (PSBMA), and quaternary ammonium units–poly([2-(acryloyloxy)ethyl]trimethylammonium chloride) (PDAC) of different proportions from a transparent cellulose membrane led to an improvement in the antifouling and antibacterial effect of the modified material (Fig. 6) (Xu et al. 2020). As the grafting ratios of PSBMA increased, a significant reduction of water contact angle from 55 to 13º was observed. More hydrophilic surfaces reduced nonspecific protein adsorption evaluated for negatively charged BSA and positively charged lysozyme (Lyz) at pH 7.4 (Fig. 6b). The inhibition of absorption was stronger for Lyz due to its net positive charge, therefore electrostatic repulsive forces between the positively charged cellulose membrane grafted with cationic PDAC and zwitterionic PSBMA, and Lyz lead to inhibition of its adsorption. While negatively charged BSA protein can be more promoted in these conditions. Additionally, modified CM exhibited antimicrobial properties against both Gram-positive and Gram-negative bacteria, up to 95.1% and 90.5% of S. aureus and E. coli reduction rates, respectively. It is worth mentioning that CM grafted with a polyelectrolyte layer demonstrated no in vitro toxicity to using a range of different target cells or cell lines.

Reproduced from Ref. (Xu et al. 2020) with permission. Copyright 2020 Elsevier Ltd
a Structural composition of antifouling, antibacterial, and non-cytotoxic cellulose membrane grafted with zwitterion and quaternary ammonium copolymers; b Adsorption of proteins (BSA and Lyz) on CMs, CM-1–PDAC modified CM, CM-2 to CM-4–CMs modified with PDAC-b-PSBMA copolymers, CM-5–PSBMA modified CM; c Synthetic route for the preparation of zwitterion and quaternary ammonium copolymers grafted from CM; d Demonstration of high transparency of the modified cellulose membranes; e Illustration of high flexibility, affinity, and adaptability to human body parts of the modified CM.
Another concept for grafting of zwitterion polymer from CM includes a direct polymerization of DMAEMA from the membrane surface followed by ring-opening reacted with beta-propiolactone to yield carboxybetaine brush (Wang et al. 2013). Carboxybetaine-based membrane exhibited improved resistance to nonspecific protein adsorption and platelet adhesion evaluated by plasma protein adsorption and platelet adhesion test, and also reduced hemolysis rate compared to unmodified CM.
Wickramasinghe et al. controlled grafting of PHEMA (Carter et al. 2018) and PGMA (Sengupta and Wickramasinghe 2020) through the AGET ATRP concept on the external cellulose membrane surface and the internal pores of the membrane utilizing various pore filling solvents (i.e. acetonitrile, ethanol, PEG 400, Pluronic L 64 and glycerol). The used solvents have a wide range of viscosity and different reactivity during the acylation of RC membrane to incorporate ATRP initiator. The immobilization of the initiator within the membrane pores was mostly suppressed using glycerol as a filling solvent. The presented concept of cellulose membrane modification demonstrates the method for controlling the location of the grafted polymer in the stage of ATRP initiator immobilization. Therefore it is possible to modify only the outer surface of the membrane, thus the properties of the material can be enhanced while minimizing the effect on membrane performance.
Summarizing the work to date, the use of a high concentration of catalyst for polymerization from the surface is not overly problematic in the context of purifying the final product in the form of a cellulose membrane grafted with various polymers. It is enough to rinse the membrane with a suitable solvent or use ultrasounds to remove unreacted substrates and a catalyst from the flat surfaces (Balis et al. 2020; Flejszar et al. 2022). On the other hand, modern polymer chemistry is heading towards the development of processes consistent with the "principles of green chemistry" to make syntheses more sustainable, therefore the use of a high concentration of a metal catalyst is thriftless and generates a lot of waste for disposal. For this reason, a number of new concepts in ATRP have been developed, which made it possible to significantly reduce the concentration of the catalyst in the reaction systems, even down to 1 ppm (Chmielarz and Sobkowiak 2017), or allowed for its elimination from the reaction mixture (Zaborniak and Chmielarz 2022; Zaborniak et al. 2020b).
Low ppm ATRP methods in cellulose membrane functionalization
Low ppm ATRP techniques were only used a few times to modify cellulose membranes utilizing copper-based catalyst concentrations of 50–10,000 ppm and ascorbic acid to reduce the deactivated form of copper (II) halide catalytic complex to its active form (Table 3) (Bhut et al. 2012; Qiu et al. 2013; Worthley et al. 2011). Analogous to AGET ATRP, an ATRP concept with activator regeneration was applied for DMAEMA (Bhut et al. 2012) and HEA (Worthley et al. 2011) polymerization from CM. The essential difference between the AGET and ARGET ATRP techniques is the amount of reducing agent used in relation to the catalyst. AGET ATRP typically uses an equimolar amount or molar insufficiency with respect to the catalyst to generate an activator (Carter et al. 2018; Sengupta and Wickramasinghe 2020; Wang et al. 2013; Xu et al. 2020), while ARGET ATRP introduces a significant excess of reductant to the catalyst into the reaction system, ensuring continuous regeneration of the activators (Bhut et al. 2012; Worthley et al. 2011). Husson et al. greatly simplified the modification of cellulose membranes to produce adsorptive material for protein binding, polymerizing DMAEMA in the reaction mixture containing only 50–200 ppm of catalyst (Bhut et al. 2012). In addition to overcoming the need for specialized catalyst handling, the proposed concept also avoided the solution deoxidation steps required for standard ATRP approaches. A significant excess of ascorbic acid in relation to the catalyst (up to 31:1) is sufficient to consume headspace oxygen in the reaction chamber, therefore, the proposed concept does not require deoxygenation of the reaction mixture or specialized preparatory equipment. Additionally, DMAEMA containing tertiary amine group serves as an internal reducing agent, therefore also consuming the oxygen in the headspace of the reaction vial (Dong and Matyjaszewski 2008; Flejszar et al. 2021). For HEMA polymerization from initiators attached to cellulose acetate membranes, tris(2-pyridylmethyl) amine (TPMA) was used as copper complexes with TPMA are more active catalysts compared to the previously mentioned and used in the classic ATRP concept (Worthley et al. 2011). The received materials were tested in the context of biofouling resistance compared to pristine CA membrane by immersion in a natural seawater aquarium with a salinity of 20,000 ppm. HEMA-modified membranes with low graft density exhibited up to 28% improvement in resistance to seawater microbial biofouling in comparison to an unmodified material, and the salt rejection and water flux decreased just by 6%, therefore the attachment of the polymer did not significantly affect the functions of the membranes.
A membrane with different characteristics on both sides was developed by simultaneously grafting NIPAM and 2-(diethylamino)ethyl methacrylate (DEAEMA) from different sides of an initiator-functionalized CM conducted in a diffusion device (Fig. 7a–e) (Qiu et al. 2013).

Reproduced from Ref. (Qiu et al. 2013) with permission. Copyright 2012 Elsevier Ltd
a Transdermal diffusion device consisted of two detachable glass chambers with two sampling ports, a stirrer, and an interlayer; XPS spectra of unmodified b CM, c brominated CM, d CM side grafted with PNIPAM, and e CM side grafted with PDEAEMA. Water contact angles for surface grafted with f PNIPAM at varying temperatures, and g PDEAEMA at different pH values. h Water contact angles determined periodically for surface grafted with PNIPAM at different temperatures: cycles from 20 to 50 °C, and for surface grafted with PDEAEMA at different pH values cycles from pH 1.0 to pH 7.0.
The polymerization was conducted in the presence of copper powder as a reducing agent and the membrane was sandwiched between two chambers, where the reaction mixtures with different monomers were introduced. The produced membrane showed individual features depending on the side. The surface grafted with PNIPAM exhibited LCST temperature–the layer became hydrophobic above LCST (Fig. 7f, h). While the PDEAEMA-based side demonstrated pH responsiveness and depending on the reaction of the environment, it was either hydrophilic or hydrophobic in acidic aqueous solution and under basic conditions, respectively (Fig. 7g, h).
Summary and outlook
Cellulose is a biobased and widely available compound, therefore the production of the membrane made of this substrate is an economic and efficient approach. Despite the high chemical and mechanical stability of the cellulose membrane, there are limited physiologically relevant or pharmaceutically clinical applications for this polymer due to its dissolution difficulty. Moreover, native cellulose membranes efficiently separate the components of the mixture only against the molecular weight of the individual components. With the rapid development of industry e.g. pharmaceutical purification, water treatment, and following synthetic routes in accordance with the principles of green chemistry, higher requirements are put forward for precision separation. Thus, the modification of cellulose membranes with polymers to improve their properties and extend their application is extensively studied, simultaneously looking for controlled grafting methods while minimizing the effect on membrane performance after modification. In this context, ATRP techniques are widely used due to excellent control over molecular weight (MW), narrow dispersity (MWD) of received polymers, and the possibility of polymerization of various types of monomers i.e. acrylates, methacrylates, and acrylamides. Cellulose membranes have been modified by classical ATRP technique in most cases. This concept uses high catalyst concentration and the catalysts must be prepared in a de-oxygenated solvent under an oxygen-free environment, and stored in an inert atmosphere. Moreover, a strictly oxygen-free environment must be maintained throughout the process. The application of high catalyst concentration is not economic, and the process of catalyst handling can be challenging, therefore classical ATRP approach may become impractical at the industrial scale. Some of these obstacles have been overcome by using the methods with catalyst regeneration, thus the metal transition catalyst was incorporated into the reaction mixture in its oxidized form in much lower concentration and then reduced in situ by an appropriate chemical reducing agent. Looking at the dynamic development of economic and effective ATRP techniques in recent years, the main direction of research on the modification of cellulose membranes should be focused mainly on concepts controlled by external factors, such as electric current, ultrasound, and especially light. Light-triggered ATRP besides simple experimental setup, mild conditions with limited side reactions, easily accessible and tunable external stimuli source, and temporal control is the only externally controlled approach providing a metal-free catalyst system. Moreover, it is possible to use naturally-derived photocatalysts eliminating harmful organic chemicals from the reaction setup. The use of the light-mediated concept is the main goal to provide efficient membrane functionalization. Furthermore, the newest achievement in RDRP techniques controlled by light enables also controlling a penetration gradient and omits limited diffusion of photocatalysts using ultrasonication waves (Wang et al. 2022b), which is crucial for uniform modification of the external cellulose membrane surface and its internal pores.
Oxygen tolerance is another direction for efficient membrane modification in microscale or large membrane surfaces for potential industrial application by ATRP. Here, several potential development directions can be cited in the context of membrane surface modification (Szczepaniak et al. 2021). There are examples of the modification of flat surfaces (silicon wafers) using a copper (Zhang et al. 2019b) or iron (Layadi et al. 2020) plate as both a catalyst source and a reducing agent. In the proposed reaction setup, polymerization was conducted between an initiator-bearing silicon wafer and a copper plate, because the microliter volumes of the reaction mixture were injected between the substrate and the plate. The limited oxygen diffusion into the system was received due to the small distance between the plates, therefore oxygen exclusion was unnecessary. Oxygen tolerance in ATRP can be achieved by using an excess of a ligand (Dadashi-Silab et al. 2020) or reducing agent (Matyjaszewski et al. 2007), which continuously regenerates the oxidized catalyst, and the monomers with tertiary amine groups can also play a role of a reducing agent e.g., DAMEMA (Dong and Matyjaszewski 2008; Zaborniak et al. 2022). The above-mentioned concepts are based on activator regeneration and can only tolerate a limited amount of oxygen and do not allow open vessel polymerization. It is also possible to use cheap, nontoxic, highly active enzymes with good thermal stability for reaction mixture deoxygenation (Sun et al. 2019). The enzyme-assisted techniques consume oxygen without interacting with the polymerization process and offer oxygen tolerance high enough to allow open vessel polymerization. The proposed concepts of oxygen tolerant reaction setups, especially photoinduced techniques, have tremendous potential for cellulose membrane modification, especially in µL-volumes, and also for modification of large surfaces.
References
Alheety MA, Majeed AH, Ali AH, Mohammed LA, Destagul A, Singh PK (2022) Synthesis and characterization of eggshell membrane polymer-TiO2 nanocomposite for newly synthesized ionic liquid release. J Iran Chem Soc 19:4005–4015. https://doi.org/10.1007/s13738-022-02584-x
Allushi A, Jockusch S, Yilmaz G, Yagci Y (2016) Photoinitiated metal-free controlled/living radical polymerization using polynuclear aromatic hydrocarbons. Macromolecules 49:7785–7792. https://doi.org/10.1021/acs.macromol.6b01752
Anastas P, Eghbali N (2010) Green chemistry: Principles and practice. Chem Soc Rev 39:301–312. https://doi.org/10.1039/B918763B
Balis A, Wolski K, Zapotoczny S (2020) Thermoresponsive polymer gating system on mesoporous shells of silica particles serving as smart nanocontainers. Polymers (basel) 12:888. https://doi.org/10.3390/polym12040888
Bayramoglu G, Arica MY (2021) Grafting of regenerated cellulose films with fibrous polymer and modified into phosphate and sulfate groups: application for removal of a model azo-dye. Colloids Surf A Physicochem Eng Asp 614:126173. https://doi.org/10.1016/j.colsurfa.2021.126173
Bhut BV, Husson SM (2009) Dramatic performance improvement of weak anion-exchange membranes for chromatographic bioseparations. J Membr Sci 337:215–223. https://doi.org/10.1016/j.memsci.2009.03.046
Bhut BV, Wickramasinghe SR, Husson SM (2008) Preparation of high-capacity, weak anion-exchange membranes for protein separations using surface-initiated atom transfer radical polymerization. J Membr Sci 325:176–183. https://doi.org/10.1016/j.memsci.2008.07.028
Bhut BV, Conrad KA, Husson SM (2012) Preparation of high-performance membrane adsorbers by surface-initiated AGET ATRP in the presence of dissolved oxygen and low catalyst concentration. J Membr Sci 390–391:43–47. https://doi.org/10.1016/j.memsci.2011.10.057
Bian C, Zhou Y-N, Guo J-K, Luo Z-H (2018) Aqueous metal-free atom transfer radical polymerization: experiments and model-based approach for mechanistic understanding. Macromolecules 51:2367–2376. https://doi.org/10.1021/acs.macromol.8b00348
Bian C, Zhou Y-N, Deetz JD, Luo Z-H (2019) Experimental and computational investigation of oxidative quenching governed aqueous organocatalyzed atom transfer radical polymerization. Chem Eng J 362:721–730. https://doi.org/10.1016/j.cej.2019.01.087
Brilmayer R, Kübelbeck S, Khalil A, Brodrecht M, Kunz U, Kleebe H-J, Buntkowsky G, Baier G, Andrieu-Brunsen A (2020) Influence of nanoconfinement on the pKa of polyelectrolyte functionalized silica mesopores. Adv Mater Interfaces 7:1901914. https://doi.org/10.1002/admi.201901914
Cao M, Liu Y, Zhang X, Li F, Zhong M (2022) Expanding the toolbox of controlled/living branching radical polymerization through simulation-informed reaction design. Chem 8:1460–1475. https://doi.org/10.1016/j.chempr.2022.02.022
Carter BM, Sengupta A, Qian X, Ulbricht M, Wickramasinghe SR (2018) Controlling external versus internal pore modification of ultrafiltration membranes using surface-initiated AGET-ATRP. J Membr Sci 554:109–116. https://doi.org/10.1016/j.memsci.2018.02.066
Chen N, Lee YM (2021) Anion exchange polyelectrolytes for membranes and ionomers. Prog Polym Sci 113:101345. https://doi.org/10.1016/j.progpolymsci.2020.101345
Chenette HCS, Robinson JR, Hobley E, Husson SM (2012) Development of high-productivity, strong cation-exchange adsorbers for protein capture by graft polymerization from membranes with different pore sizes. J Membr Sci 423–424:43–52. https://doi.org/10.1016/j.memsci.2012.07.040
Chmielarz P (2016) Synthesis of cationic star polymers by simplified electrochemically mediated ATRP. Express Polym Lett 10:810–821. https://doi.org/10.3144/expresspolymlett.2016.76
Chmielarz P (2017) Synthesis of pyridoxine-based eagle-shaped asymmetric star polymers through seATRP. Polym Adv Technol 28:1787–1793. https://doi.org/10.1002/pat.4062
Chmielarz P (2018) Synthesis of naringin-based polymer brushes via seATRP. Polym Adv Technol 29:470–480. https://doi.org/10.1002/pat.4136
Chmielarz P, Sobkowiak A (2017) Ultralow ppm seATRP synthesis of PEO-b-PBA copolymers. J Polym Res 24:77. https://doi.org/10.1007/s10965-017-1235-2
Chmielarz P, Krys P, Park S, Matyjaszewski K (2015a) PEO-b-PNIPAM copolymers via SARA ATRP and eATRP in aqueous media. Polymer 71:143–147. https://doi.org/10.1016/j.polymer.2015.06.042
Chmielarz P, Park S, Simakova A, Matyjaszewski K (2015b) Electrochemically mediated ATRP of acrylamides in water. Polymer 60:302–307. https://doi.org/10.1016/j.polymer.2015.01.051
Chmielarz P, Fantin M, Park S, Isse AA, Gennaro A, Magenau AJD, Sobkowiak A, Matyjaszewski K (2017a) Electrochemically mediated atom transfer radical polymerization (eATRP). Prog Polym Sci 69:47–78. https://doi.org/10.1016/j.progpolymsci.2017.02.005
Chmielarz P, Krys P, Wang Z, Wang Y, Matyjaszewski K (2017b) Synthesis of well-defined polymer brushes from silicon wafers via surface-initiated seATRP. Macromol Chem Phys 218:1700106. https://doi.org/10.1002/macp.201700106
Chmielarz P, Paczesniak T, Rydel-Ciszek K, Zaborniak I, Biedka P, Sobkowiak A (2017c) Synthesis of naturally-derived macromolecules through simplified electrochemically mediated ATRP. Beilstein J Org Chem 13:2466–2472. https://doi.org/10.3762/bjoc.13.243
Chmielarz P, Yan J, Krys P, Wang Y, Wang Z, Bockstaller MR, Matyjaszewski K (2017d) Synthesis of nanoparticle copolymer brushes via surface-initiated seATRP. Macromolecules 50:4151–4159. https://doi.org/10.1021/acs.macromol.7b00280
Corbin DA, Miyake GM (2022) Photoinduced organocatalyzed atom transfer radical polymerization (O-ATRP): precision polymer synthesis using organic photoredox catalysis. Chem Rev 122:1830–1874. https://doi.org/10.1021/acs.chemrev.1c00603
Dadashi-Silab S, Lee I-H, Anastasaki A, Lorandi F, Narupai B, Dolinski ND, Allegrezza ML, Fantin M, Konkolewicz D, Hawker CJ, Matyjaszewski K (2020) Investigating temporal control in photoinduced atom transfer radical polymerization. Macromolecules 53:5280–5288. https://doi.org/10.1021/acs.macromol.0c00888
De Bon F, Fonseca RG, Lorandi F, Serra AC, Isse AA, Matyjaszewski K, Coelho JFJ (2022) The scale-up of electrochemically mediated atom transfer radical polymerization without deoxygenation. Chem Eng J 445:136690. https://doi.org/10.1016/j.cej.2022.136690
Discekici EH, Pester CW, Treat NJ, Lawrence J, Mattson KM, Narupai B, Toumayan EP, Luo Y, McGrath AJ, Clark PG, Read de Alaniz J, Hawker CJ (2016) Simple benchtop approach to polymer brush nanostructures using visible-light-mediated metal-free atom transfer radical polymerization. ACS Macro Lett 5:258–262. https://doi.org/10.1021/acsmacrolett.6b00004
Discekici EH, Shankel SL, Anastasaki A, Oschmann B, Lee I-H, Niu J, McGrath AJ, Clark PG, Laitar DS, de Alaniz JR, Hawker CJ, Lunn DJ (2017) Dual-pathway chain-end modification of RAFT polymers using visible light and metal-free conditions. Chem Commun 53:1888–1891. https://doi.org/10.1039/C6CC08370F
Discekici EH, Anastasaki A, Read de Alaniz J, Hawker CJ (2018) Evolution and future directions of metal-free atom transfer radical polymerization. Macromolecules 51:7421–7434. https://doi.org/10.1021/acs.macromol.8b01401
Dong H, Matyjaszewski K (2008) ARGET ATRP of 2-(dimethylamino)ethyl methacrylate as an intrinsic reducing agent. Macromolecules 41:6868–6870. https://doi.org/10.1021/ma8017553
Dworakowska S, Lorandi F, Gorczyński A, Matyjaszewski K (2022) Toward green atom transfer radical polymerization: current status and future challenges. Adv Sci 9:2106076. https://doi.org/10.1002/advs.202106076
Eduok U, Abdelrasoul A, Shoker A, Doan H (2021) Recent developments, current challenges and future perspectives on cellulosic hemodialysis membranes for highly efficient clearance of uremic toxins. Mater Today Commun 27:102183. https://doi.org/10.1016/j.mtcomm.2021.102183
Feng Q, Hou D, Zhao Y, Xu T, Menkhaus TJ, Fong H (2014) Electrospun regenerated cellulose nanofibrous membranes surface-grafted with olymer chains/brushes via the atom transfer radical polymerization method for catalase Immobilization. ACS Appl Mater Interfaces 6:20958–20967. https://doi.org/10.1021/am505722g
Flejszar M, Chmielarz P (2019) Surface-initiated atom transfer radical polymerization for the preparation of well-defined organic–inorganic hybrid nanomaterials. Materials 12:3030. https://doi.org/10.3390/ma12183030
Flejszar M, Chmielarz P, Smenda J, Wolski K (2021) Following principles of green chemistry: low ppm photo-ATRP of DMAEMA in water/ethanol mixture. Polymer 228:123905. https://doi.org/10.1016/j.polymer.2021.123905
Flejszar M, Ślusarczyk K, Chmielarz P, Wolski K, Isse AA, Gennaro A, Wytrwal-Sarna M, Oszajca M (2022) Working electrode geometry effect: A new concept for fabrication of patterned polymer brushes via SI-seATRP at ambient conditions. Polymer 255:125098. https://doi.org/10.1016/j.polymer.2022.125098
Fu X, Lu Z, Yang H, Yin X, Xiao L, Hou L (2021) Imine-based covalent organic framework as photocatalyst for visible-light-induced atom transfer radical polymerization. J Polym Sci 59:2036–2044. https://doi.org/10.1002/pol.20210261
Fung AK, Coote ML (2021) A mechanistic perspective on atom transfer radical polymerization. Polym Int 70:918–926. https://doi.org/10.1002/pi.6130
Grześ G, Wolski K, Uchacz T, Bała J, Louis B, Scheblykin IG, Zapotoczny S (2022) Ladder-like polymer brushes containing conjugated poly(propylenedioxythiophene) chains. Int J Mol Sci 23:5886. https://doi.org/10.3390/ijms23115886
Han X, Zhang X, Zhu H, Yin Q, Liu H, Hu Y (2013) Effect of composition of PDMAEMA-b-PAA block copolymers on their pH- and temperature-responsive behaviors. Langmuir 29:1024–1034. https://doi.org/10.1021/la3036874
Han Z, Zhou Y, Yang Y, Sun Y, Wang Y, Wang Y, Sun Y (2022) Preparation of myoglobin-imprinted polymer with a new sulphoxide monomer on the surface of foam-graphene/nano Au via eATRP. Bull Mater Sci 45:58. https://doi.org/10.1007/s12034-021-02640-x
Himstedt HH, Qian X, Weaver JR, Wickramasinghe SR (2013) Responsive membranes for hydrophobic interaction chromatography. J Membr Sci 447:335–344. https://doi.org/10.1016/j.memsci.2013.07.020
Hu X, Wei W, Qi X, Yu H, Feng L, Li J, Wang S, Zhang J, Dong W (2015) Preparation and characterization of a novel pH-sensitive Salecan-g-poly(acrylic acid) hydrogel for controlled release of doxorubicin. J Mater Chem B 3:2685–2697. https://doi.org/10.1039/C5TB00264H
Huang ZC, Gu Y, Liu XD, Zhang LF, Cheng ZP, Zhu XL (2017) Metal-free atom transfer radical polymerization of methyl methacrylate with ppm level of organic photocatalyst. Macromol Rapid Commun 38:1600461. https://doi.org/10.1002/marc.201600461
Jain K, Vedarajan R, Watanabe M, Ishikiriyama M, Matsumi N (2015) Tunable LCST behavior of poly(N-isopropylacrylamide/ionic liquid) copolymers. Polym Chem 6:6819–6825. https://doi.org/10.1039/C5PY00998G
Jiang M, Wang J, Li L, Pan K, Cao B (2013) Poly(N,N-dimethylaminoethyl methacrylate) modification of a regenerated cellulose membrane using ATRP method for copper(II) ion removal. RSC Adv 3:20625–20632. https://doi.org/10.1039/C3RA44126A
Kang C, Crockett RM, Spencer ND (2014) Molecular-weight determination of polymer brushes generated by SI-ATRP on flat surfaces. Macromolecules 47:269–275. https://doi.org/10.1021/ma401951w
Kang H, Liu R, Huang Y (2015) Graft modification of cellulose: Methods, properties and applications. Polymer 70:A1–A16. https://doi.org/10.1016/j.polymer.2015.05.041
Khan B, Zhan W, Lina C (2020) Cellulose acetate (CA) hybrid membrane prepared by phase inversion method combined with chemical reaction with enhanced permeability and good anti-fouling property. J Appl Polym Sci 137:49556. https://doi.org/10.1002/app.49556
Konkolewicz D, Magenau AJD, Averick SE, Simakova A, He H, Matyjaszewski K (2012) ICAR ATRP with ppm Cu catalyst in water. Macromolecules 45:4461–4468. https://doi.org/10.1021/ma300887r
Konkolewicz D, Wang Y, Zhong MJ, Krys P, Isse AA, Gennaro A, Matyjaszewski K (2013) Reversible-deactivation radical polymerization in the presence of metallic copper. a critical assessment of the SARA ATRP and SET-LRP mechanisms. Macromolecules 46:8749–8772. https://doi.org/10.1021/ma401243k
Konkolewicz D, Wang Y, Krys P, Zhong M, Isse AA, Gennaro A, Matyjaszewski K (2014) SARA ATRP or SET-LRP. End of controversy? Polym Chem 5:4396–4417. https://doi.org/10.1039/C4PY00149D
Krys P, Matyjaszewski K (2017) Kinetics of atom transfer radical polymerization. Eur Polym J 89:482–523. https://doi.org/10.1016/j.eurpolymj.2017.02.034
Layadi A, Kessel B, Yan W, Romio M, Spencer ND, Zenobi-Wong M, Matyjaszewski K, Benetti EM (2020) Oxygen tolerant and cytocompatible iron(0)-mediated ATRP enables the controlled growth of polymer brushes from mammalian cell cultures. J Am Chem Soc 142:3158–3164. https://doi.org/10.1021/jacs.9b12974
Liu L, Yi Y (2018) Photo-mediated metal free atom transfer radical polymerization of acrylamide in water. J Appl Polym Sci 135:46567. https://doi.org/10.1002/app.46567
Liu P-S, Chen Q, Liu X, Yuan B, Wu S-S, Shen J, Lin S-C (2009) Grafting of zwitterion from cellulose membranes via ATRP for improving blood compatibility. Biomacromol 10:2809–2816. https://doi.org/10.1021/bm9006503
Liu P-S, Chen Q, Wu S-S, Shen J, Lin S-C (2010) Surface modification of cellulose membranes with zwitterionic polymers for resistance to protein adsorption and platelet adhesion. J Membr Sci 350:387–394. https://doi.org/10.1016/j.memsci.2010.01.015
Liu P, Chen Q, Li L, Lin S, Shen J (2014) Anti-biofouling ability and cytocompatibility of the zwitterionic brushes-modified cellulose membrane. J Mater Chem B 2:7222–7231. https://doi.org/10.1039/C4TB01151A
Liu Y-X, Bian C, Zhou Y-N, Li J-J, Luo Z-H (2020) Kinetic study on ultraviolet light-induced solution atom transfer radical polymerization of methyl acrylate using TiO2. Ind Eng Chem Res 59:13870–13878. https://doi.org/10.1021/acs.iecr.0c01534
Liu K, Zhang W, Zong L, He Y, Zhang X, Liu M, Shi G, Qiao X, Pang X (2022a) Dimensional optimization for ZnO-based mechano-ATRP with extraordinary activity. J Phys Chem Lett 13:4884–4890. https://doi.org/10.1021/acs.jpclett.2c01106
Liu Y, Li S, Wang Z, Wang L (2022b) Ultrasound in cellulose-based hydrogel for biomedical use: from extraction to preparation. Colloids Surf, B 212:112368. https://doi.org/10.1016/j.colsurfb.2022.112368
Lorandi F, Fantin M, Wang Y, Isse AA, Gennaro A, Matyjaszewski K (2020) Atom transfer radical polymerization of acrylic and methacrylic acids: preparation of acidic polymers with various architectures. ACS Macro Lett 9:693–699. https://doi.org/10.1021/acsmacrolett.0c00246
Lu CW, Wang CP, Yu J, Wang JF, Chu FX (2019) Metal- free ATRP “ grafting from” technique for renewable cellulose graft copolymers. Green Chem 21:2759–2770. https://doi.org/10.1039/c9gc00138g
Luan M, Shen D, Zhou P, Li D, Li P, Shi B, Wang G (2022) One-pot synthesis of block copolymer dispersant by ICAR ATRP with ppm copper catalyst and the dispersibility on pigment. Prog Org Coat 169:106914. https://doi.org/10.1016/j.porgcoat.2022.106914
Luo J, Durante C, Gennaro A, Isse AA (2021) Electrochemical study of the effect of Al3+ on the stability and performance of Cu-based ATRP catalysts in organic media. Electrochim Acta 388:138589. https://doi.org/10.1016/j.electacta.2021.138589
Luo Y, Li Z, Chen Y, Jin T (2022) Esterification process of incorporation CS2 activation and its effect on the properties of hydrophobic modified cotton filter fabric via ARGET-ATRP. Eur Polym J 168:111092. https://doi.org/10.1016/j.eurpolymj.2022.111092
Macior A, Zaborniak I, Chmielarz P, Smenda J, Wolski K, Ciszkowicz E, Lecka-Szlachta K (2022) A new protocol for ash wood modification: synthesis of hydrophobic and antibacterial brushes from the wood surface. Molecules 27:890. https://doi.org/10.3390/molecules27030890
Maeda Y, Nakamura T, Ikeda I (2002) Hydration and phase behavior of poly(N-vinylcaprolactam) and poly(N-vinylpyrrolidone) in water. Macromolecules 35:217–222. https://doi.org/10.1021/ma011034+
Matyjaszewski K (2012a) Atom transfer radical polymerization (ATRP): current status and future perspectives. Macromolecules 45:4015–4039. https://doi.org/10.1021/Ma3001719
Matyjaszewski K (2012b) Atom transfer radical polymerization: from mechanisms to applications. Isr J Chem 52:206–220. https://doi.org/10.1002/ijch.201100101
Matyjaszewski K (2018) Advanced materials by atom transfer radical polymerization. Adv Mater 30:1706441. https://doi.org/10.1002/adma.201706441
Matyjaszewski K, Dong H, Jakubowski W, Pietrasik J, Kusumo A (2007) Grafting from surfaces for “everyone”: ARGET ATRP in the presence of air. Langmuir 23:4528–4531. https://doi.org/10.1021/la063402e
Menkhaus TJ, Varadaraju H, Zhang L, Schneiderman S, Bjustrom S, Liu L, Fong H (2010) Electrospun nanofiber membranes surface functionalized with 3-dimensional nanolayers as an innovative adsorption medium with ultra-high capacity and throughput. Chem Commun 46:3720–3722. https://doi.org/10.1039/C001802C
Min K, Gao H, Matyjaszewski K (2007) Use of ascorbic acid as reducing agent for synthesis of well-defined polymers by ARGET ATRP. Macromolecules 40:1789–1791. https://doi.org/10.1021/ma0702041
Mohapatra H, Kleiman M, Esser-Kahn AP (2017) Mechanically controlled radical polymerization initiated by ultrasound. Nat Chem 9:135–139. https://doi.org/10.1038/nchem.2633
Mohapatra H, Ayarza J, Sanders EC, Scheuermann AM, Griffin PJ, Esser-Kahn AP (2018) Ultrasound promoted step-growth polymerization and polymer crosslinking via copper catalyzed azide–alkyne “click” reaction. Angew Chem Int Ed 57:11208–11212. https://doi.org/10.1002/anie.201804451
Muzammil Ezzah M, Khan A, Stuparu MC (2017) Post-polymerization modification reactions of poly(glycidyl methacrylate)s. RSC Adv 7:55874–55884. https://doi.org/10.1039/C7RA11093F
O’Brien C, Ignaszak A (2022) Polymer-grafted-carbon assembled via an electrochemically-aided atom transfer radical polymerization: towards improved energy storage electrode. Electrochem Commun 135:107198. https://doi.org/10.1016/j.elecom.2021.107198
Pan K, Zhang X, Cao B (2010a) Surface-initiated atom transfer radical polymerization of regenerated cellulose membranes with thermo-responsive properties. Polym Int 59:733–737. https://doi.org/10.1002/pi.2773
Pan K, Zhang X, Ren R, Cao B (2010b) Double stimuli-responsive membranes grafted with block copolymer by ATRP method. J Membr Sci 356:133–137. https://doi.org/10.1016/j.memsci.2010.03.044
Pan K, Zhang X, Zhu J, Cao B (2011) Grafting of regenerated cellulose membrane by surface-initiated atom transfer radical polymerization and its pH-resposive behavior. Polym Adv Technol 22:1948–1952. https://doi.org/10.1002/pat.1699
Pan X, Fantin M, Yuan F, Matyjaszewski K (2018) Externally controlled atom transfer radical polymerization. Chem Soc Rev 47:5457–5490. https://doi.org/10.1039/C8CS00259B
Park S, Chmielarz P, Gennaro A, Matyjaszewski K (2015) Simplified electrochemically mediated atom transfer radical polymerization using a sacrificial anode. Angew Chem Int Ed 54:2388–2392. https://doi.org/10.1002/anie.201410598
Park GS, Back J, Choi EM, Lee E, Son K-s (2019) Visible light-mediated metal-free atom transfer radical polymerization with N-trifluoromethylphenyl phenoxazines. Eur Polym J 117:347–352. https://doi.org/10.1016/j.eurpolymj.2019.05.023
Parkatzidis K, Wang HS, Truong NP, Anastasaki A (2020) Recent developments and future challenges in controlled radical polymerization: A 2020 update. Chem 6:1575–1588. https://doi.org/10.1016/j.chempr.2020.06.014
Porter CJ, Werber JR, Ritt CL, Guan Y-F, Zhong M, Elimelech M (2020) Controlled grafting of polymer brush layers from porous cellulosic membranes. J Membr Sci 596:117719. https://doi.org/10.1016/j.memsci.2019.117719
Porter CJ, DuChanois RM, MacDonald E, Kilpatrick S-M, Zhong M, Elimelech M (2022) Tethered electrolyte active-layer membranes. J Membr Sci 642:120004. https://doi.org/10.1016/j.memsci.2021.120004
Pu H, Xu L (2022) Molecularly imprinted nanoparticles synthesized by electrochemically mediated atom transfer radical precipitation polymerization. Macromol Chem Phys 223:2100478. https://doi.org/10.1002/macp.202100478
Qian X, Fan H, Wang C, Wei Y (2013) Preparation of high-capacity, weak anion-exchange membranes by surface-initiated atom transfer radical polymerization of poly(glycidyl methacrylate) and subsequent derivatization with diethylamine. Appl Surf Sci 271:240–247. https://doi.org/10.1016/j.apsusc.2013.01.167
Qiao L, Zhou M, Shi G, Cui Z, Zhang X, Fu P, Liu M, Qiao X, He Y, Pang X (2022) Ultrafast visible-light-induced ATRP in aqueous media with carbon quantum dots as the catalyst and its application for 3D printing. J Am Chem Soc 144:9817–9826. https://doi.org/10.1021/jacs.2c02303
Qiu X, Ren X, Hu S (2013) Fabrication of dual-responsive cellulose-based membrane via simplified surface-initiated ATRP. Carbohydr Polym 92:1887–1895. https://doi.org/10.1016/j.carbpol.2012.11.080
Razeghi R, Kazemi F, Nikfarjam N, Shariati Y, Kaboudin B (2021) Visible photo-induced catalyst-free polymerization via in situ prepared dibromide. Eur Polym J 144:110195. https://doi.org/10.1016/j.eurpolymj.2020.110195
Sengupta A, Wickramasinghe R (2020) Activator generated electron transfer combined atom transfer radical polymerization (AGET-ATRP) for controlled grafting location of glycidyl methacrylate on regenerated cellulose ultrafiltration membranes. J Membr Sci Res 6:90–98. https://doi.org/10.22079/jmsr.2019.109047.1266
Shokri M, Moradi S, Amini S, Shahlaei M, Seidi F, Saedi S (2021) A novel amino cellulose derivative using ATRP method: preparation, characterization, and investigation of its antibacterial activity. Bioorg Chem 106:104355. https://doi.org/10.1016/j.bioorg.2020.104355
Singh N, Chen Z, Tomer N, Wickramasinghe SR, Soice N, Husson SM (2008a) Modification of regenerated cellulose ultrafiltration membranes by surface-initiated atom transfer radical polymerization. J Membr Sci 311:225–234. https://doi.org/10.1016/j.memsci.2007.12.036
Singh N, Wang J, Ulbricht M, Wickramasinghe SR, Husson SM (2008b) Surface-initiated atom transfer radical polymerization: a new method for preparation of polymeric membrane adsorbers. J Membr Sci 309:64–72. https://doi.org/10.1016/j.memsci.2007.10.007
Soly S, Mistry B, Murthy C (2022) Photo-mediated metal-free atom transfer radical polymerization: recent advances in organocatalysts and perfection towards polymer synthesis. Polym Int 71:159–168. https://doi.org/10.1002/pi.6336
Su H-L, Yang M-M, Liu M, Fu J-W, Wang Y-H, Yao M-X, Yang D-H, Wang L-P, Li G (2022) PH and thermo dual-sensitive copolymers with fluorescent properties grafted mesoporous silica SBA-15 via metal-free ATRP. Eur Polym J 167:111064. https://doi.org/10.1016/j.eurpolymj.2022.111064
Sun Y, Lathwal S, Wang Y, Fu L, Olszewski M, Fantin M, Enciso AE, Szczepaniak G, Das S, Matyjaszewski K (2019) Preparation of well-defined polymers and DNA–polymer bioconjugates via small-volume eATRP in the presence of air. ACS Macro Lett 8:603–609. https://doi.org/10.1021/acsmacrolett.9b00159
Szczepaniak G, Fu L, Jafari H, Kapil K, Matyjaszewski K (2021) Making ATRP more practical: oxygen tolerance. Acc Chem Res 54:1779–1790. https://doi.org/10.1021/acs.accounts.1c00032
Tasdelen MA, Uygun M, Yagci Y (2011) Photoinduced controlled radical polymerization. Macromol Rapid Commun 32:58–62. https://doi.org/10.1002/marc.201000351
Thakur VK, Voicu SI (2016) Recent advances in cellulose and chitosan based membranes for water purification: a concise review. Carbohydr Polym 146:148–165. https://doi.org/10.1016/j.carbpol.2016.03.030
Theriot JC, Lim C-H, Yang H, Ryan MD, Musgrave CB, Miyake GM (2016) Organocatalyzed atom transfer radical polymerization driven by visible light. Science 352:1082–1086. https://doi.org/10.1126/science.aaf3935
Treat NJ, Sprafke H, Kramer JW, Clark PG, Barton BE, Read de Alaniz J, Fors BP, Hawker CJ (2014) Metal-free atom transfer radical polymerization. J Am Chem Soc 136:16096–16101. https://doi.org/10.1021/ja510389m
Truong NP, Jones GR, Bradford KGE, Konkolewicz D, Anastasaki A (2021) A comparison of RAFT and ATRP methods for controlled radical polymerization. Nat Rev Chem 5:859–869. https://doi.org/10.1038/s41570-021-00328-8
Tu H, Zhu M, Duan B, Zhang L (2021) Recent progress in high-strength and robust regenerated cellulose materials. Adv Mater 33:2000682. https://doi.org/10.1002/adma.202000682
Vatanpour V, Pasaoglu ME, Barzegar H, Teber OO, Kaya R, Bastug M, Khataee A, Koyuncu I (2022) Cellulose acetate in fabrication of polymeric membranes: a review. Chemosphere 295:133914. https://doi.org/10.1016/j.chemosphere.2022.133914
Wandera D, Wickramasinghe SR, Husson SM (2011) Modification and characterization of ultrafiltration membranes for treatment of produced water. J Membr Sci 373:178–188. https://doi.org/10.1016/j.memsci.2011.03.010
Wandera D, Himstedt HH, Marroquin M, Wickramasinghe SR, Husson SM (2012) Modification of ultrafiltration membranes with block copolymer nanolayers for produced water treatment: The roles of polymer chain density and polymerization time on performance. J Membr Sci 403–404:250–260. https://doi.org/10.1016/j.memsci.2012.02.061
Wang Q, Zhang J, Wang A (2009) Preparation and characterization of a novel pH-sensitive chitosan-g-poly(acrylic acid)/attapulgite/sodium alginate composite hydrogel bead for controlled release of diclofenac sodium. Carbohydr Polym 78:731–737. https://doi.org/10.1016/j.carbpol.2009.06.010
Wang M, Yuan J, Huang X, Cai X, Li L, Shen J (2013) Grafting of carboxybetaine brush onto cellulose membranes via surface-initiated ARGET-ATRP for improving blood compatibility. Colloids Surf, B 103:52–58. https://doi.org/10.1016/j.colsurfb.2012.10.025
Wang J, Sproul RT, Anderson LS, Husson SM (2014) Development of multimodal membrane adsorbers for antibody purification using atom transfer radical polymerization. Polymer 55:1404–1411. https://doi.org/10.1016/j.polymer.2013.12.023
Wang J, Jenkins EW, Robinson JR, Wilson A, Husson SM (2015) A new multimodal membrane adsorber for monoclonal antibody purifications. J Membr Sci 492:137–146. https://doi.org/10.1016/j.memsci.2015.05.013
Wang Z, Crandall C, Prautzsch VL, Sahadevan R, Menkhaus TJ, Fong H (2017) Electrospun regenerated cellulose nanofiber membranes surface-grafted with water-insoluble poly(HEMA) or water-soluble poly(AAS) chains via the ATRP method for ultrafiltration of water. ACS Appl Mater Interfaces 9:4272–4278. https://doi.org/10.1021/acsami.6b16116
Wang Z, Wang Z, Pan X, Fu L, Lathwal S, Olszewski M, Yan J, Enciso AE, Wang Z, Xia H, Matyjaszewski K (2018) Ultrasonication-induced aqueous atom transfer radical polymerization. ACS Macro Lett 7:275–280. https://doi.org/10.1021/acsmacrolett.8b00027
Wang Z, Ayarza J, Esser-Kahn AP (2019) Mechanically initiated bulk-scale free-radical polymerization. Angew Chem Int Ed 58:12023–12026. https://doi.org/10.1002/anie.201903956
Wang J, Song H, Ren L, Talukder ME, Chen S, Shao J (2022a) Study on the preparation of cellulose acetate separation membrane and new adjusting method of pore size. Membranes 12:9. https://doi.org/10.3390/membranes12010009
Wang Y, Bian C, Feng W, Yang N (2022b) Ultrasonication enhanced photocatalytic solvent-free reversible deactivation radical polymerization up to high conversion with good control. Eur Polym J 168:111118. https://doi.org/10.1016/j.eurpolymj.2022.111118
Wei Y-T, Zheng Y-M, Chen JP (2011) Functionalization of regenerated cellulose membrane via surface initiated atom transfer radical polymerization for boron removal from aqueous solution. Langmuir 27:6018–6025. https://doi.org/10.1021/la200154y
Wei D, Li H, Yang C, Fu J, Chen H, Bai L, Wang W, Yang H, Yang L, Liang Y (2022) Visible light-driven acridone catalysis for atom transfer radical polymerization. J Polym Sci 60:1588–1594. https://doi.org/10.1002/pol.20210743
Williams VA, Ribelli TG, Chmielarz P, Park S, Matyjaszewski K (2015) A silver bullet: Elemental silver as an efficient reducing agent for atom transfer radical polymerization of acrylates. J Am Chem Soc 137:1428–1431. https://doi.org/10.1021/ja512519j
Worthley CH, Constantopoulos KT, Ginic-Markovic M, Pillar RJ, Matisons JG, Clarke S (2011) Surface modification of commercial cellulose acetate membranes using surface-initiated polymerization of 2-hydroxyethyl methacrylate to improve membrane surface biofouling resistance. J Membr Sci 385–386:30–39. https://doi.org/10.1016/j.memsci.2011.09.017
Xu C, Jiang J, Oguzlu H, Zheng Y, Jiang F (2020) Antifouling, antibacterial and non-cytotoxic transparent cellulose membrane with grafted zwitterion and quaternary ammonium copolymers. Carbohydr Polym 250:116960. https://doi.org/10.1016/j.carbpol.2020.116960
Xu X, Xu X, Zeng Y, Zhang F (2021) Oxygen-tolerant photo-induced metal-free atom transfer radical polymerization. J Photochem Photobiol a: Chem 411:113191. https://doi.org/10.1016/j.jphotochem.2021.113191
Xu X, Peng B, Hong M, Wang T, Fan L, Bao C, Zhang Q (2022) Photo-induced atom transfer radical polymerization of styrene using a highly active claw-type Schiff-base ligand. Eur Polym J 172:111201. https://doi.org/10.1016/j.eurpolymj.2022.111201
Yan W, Dadashi-Silab S, Matyjaszewski K, Spencer ND, Benetti EM (2020) Surface-initiated photoinduced ATRP: mechanism, oxygen tolerance, and temporal control during the synthesis of polymer brushes. Macromolecules 53:2801–2810. https://doi.org/10.1021/acs.macromol.0c00333
Yang X, Li N, Constantinesco I, Yu K, Kizhakkedathu JN, Brooks DE (2016) Choline phosphate functionalized cellulose membrane: a potential hemostatic dressing based on a unique bioadhesion mechanism. Acta Biomater 40:212–225. https://doi.org/10.1016/j.actbio.2016.06.030
Ye J, Chu J, Yin J, Zhang Y, Meng J (2020) Surface modification of regenerated cellulose membrane based on thiolactone chemistry–a novel platform for mixed mode membrane adsorbers. Appl Surf Sci 511:145539. https://doi.org/10.1016/j.apsusc.2020.145539
Yin R, Wang Z, Bockstaller MR, Matyjaszewski K (2021) Tuning dispersity of linear polymers and polymeric brushes grown from nanoparticles by atom transfer radical polymerization. Polym Chem 12:6071–6082. https://doi.org/10.1039/D1PY01178B
Yu Y, Wu Q-Y, Liang H-Q, Gu L, Xu Z-K (2017) Preparation and characterization of cellulose triacetate membranes via thermally induced phase separation. J Appl Polym Sci 134:44454. https://doi.org/10.1002/app.44454
Zaborniak I, Chmielarz P (2019a) Temporally controlled ultrasonication-mediated atom transfer radical polymerization in miniemulsion. Macromol Chem Phys 220:1900285. https://doi.org/10.1002/macp.201900285
Zaborniak I, Chmielarz P (2019b) Ultrasound-mediated atom transfer radical polymerization (ATRP). Materials 12:3600. https://doi.org/10.3390/ma12213600
Zaborniak I, Chmielarz P (2022) Comestible curcumin: from kitchen to polymer chemistry as a photocatalyst in metal-free ATRP of (meth)acrylates. J Ind Eng Chem 105:481–490. https://doi.org/10.1016/j.jiec.2021.10.001
Zaborniak I, Chmielarz P, Matyjaszewski K (2019a) Modification of wood-based materials by atom transfer radical polymerization methods. Eur Polym J 120:109253. https://doi.org/10.1016/j.eurpolymj.2019.109253
Zaborniak I, Chmielarz P, Wolski K, Grześ G, Isse AA, Gennaro A, Zapotoczny S, Sobkowiak A (2019b) Tannic acid-inspired star-like macromolecules via temporally controlled multi-step potential electrolysis. Macromol Chem Phys 220:1900073. https://doi.org/10.1002/macp.201900073
Zaborniak I, Chmielarz P, Flejszar M, Surmacz K, Ostatek R (2020a) Preparation of hydrophobic tannins-inspired polymer materials via low-ppm ATRP methods. Polym Adv Technol 31:913–921. https://doi.org/10.1002/pat.4825
Zaborniak I, Chmielarz P, Wolski K (2020b) Riboflavin-induced metal-free ATRP of (meth)acrylates. Eur Polym J 140:110055. https://doi.org/10.1016/j.eurpolymj.2020.110055
Zaborniak I, Macior A, Chmielarz P, Caceres Najarro M, Iruthayaraj J (2021a) Lignin-based thermoresponsive macromolecules via vitamin-induced metal-free ATRP. Polymer 219:123537. https://doi.org/10.1016/j.polymer.2021.123537
Zaborniak I, Macior A, Chmielarz P, Smenda J, Wolski K (2021b) Hydrophobic modification of fir wood surface via low ppm ATRP strategy. Polymer 228:123942. https://doi.org/10.1016/j.polymer.2021.123942
Zaborniak I, Sroka M, Chmielarz P (2022) Lemonade as a rich source of antioxidants: polymerization of 2-(dimethylamino)ethyl methacrylate in lemon extract. Polymer 254:125099. https://doi.org/10.1016/j.polymer.2022.125099
Zeng S, Shi J, Feng A, Wang Z (2021) Modification of electrospun regenerate cellulose nanofiber membrane via atom transfer radical polymerization (ATRP) approach as advanced carrier for laccase immobilization. Polymers (basel) 13:182. https://doi.org/10.3390/polym13020182
Zhang L, Zhao Y-H, Bai R (2011) Development of a multifunctional membrane for chromatic warning and enhanced adsorptive removal of heavy metal ions: application to cadmium. J Membr Sci 379:69–79. https://doi.org/10.1016/j.memsci.2011.05.044
Zhang S, Kai C, Liu B, Zhang S, Wei W, Xu X, Zhou Z (2019a) Preparation, characterization and antibacterial properties of cellulose membrane containing N-halamine. Cellulose 26:5621–5633. https://doi.org/10.1007/s10570-019-02492-z
Zhang T, Benetti EM, Jordan R (2019b) Surface-Initiated Cu(0)-mediated CRP for the rapid and controlled synthesis of quasi-3D structured polymer brushes. ACS Macro Lett 8:145–153. https://doi.org/10.1021/acsmacrolett.8b00912
Zhang Z, Sèbe G, Hou Y, Wang J, Huang J, Zhou G (2021) Grafting polymers from cellulose nanocrystals via surface-initiated atom transfer radical polymerization. J Appl Polym Sci 138:51458. https://doi.org/10.1002/app.51458
Zhao Y-H, Wee K-H, Bai R (2010) A novel electrolyte-responsive membrane with tunable permeation selectivity for protein purification. ACS Appl Mater Interfaces 2:203–211. https://doi.org/10.1021/am900654d
Zhao B, Mohammed M, Jones BA, Wilson P (2021) Plug-and-play aqueous electrochemical atom transfer radical polymerization. Chem Commun 57:3897–3900. https://doi.org/10.1039/D1CC01312B
Zhao B, Pashley-Johnson F, Jones BA, Wilson P (2022) Aqueous electrochemically-triggered atom transfer radical polymerization. Chem Sci 13:5741–5749. https://doi.org/10.1039/D2SC01832B
Zhou Y-N, Li J-J, Wu Y-Y, Luo Z-H (2020) Role of external field in polymerization: mechanism and kinetics. Chem Rev 120:2950–3048. https://doi.org/10.1021/acs.chemrev.9b00744
Zhou Y-N, Li J-J, Wang T-T, Wu Y-Y, Luo Z-H (2022) Precision polymer synthesis by controlled radical polymerization: Fusing the progress from polymer chemistry and reaction engineering. Prog Polym Sci 130:101555. https://doi.org/10.1016/j.progpolymsci.2022.101555
Zugenmaier P (2001) Conformation and packing of various crystalline cellulose fibers. Prog Polym Sci 26:1341–1417. https://doi.org/10.1016/S0079-6700(01)00019-3
Acknowledgments
P.C. acknowledges Minister of Science and Higher Education scholarship for outstanding young scientists (Grant Number 0001/E-363/STYP/13/2018) and National Science Centre in Poland for the financial support as a part of the SONATA BIS 10 project (Grant Number 2020/38/E/ST4/00046). I.Z. acknowledges Minister of Science and Higher Education scholarship for outstanding young scientists (Grant Number SMN/16/0615/2020). Financial support from Minister of Science and Higher Education as a part of "Student science clubs create innovation" program (Grant Number SKN/SP/534777/2022) is gratefully acknowledged.
Funding
This study has no financial interest.
Author information
Authors and Affiliations
Contributions
IZ: Conceptualization, Writing—original draft, Writing—review & editing, Visualization, Project administration, Funding acquisition. PC: Conceptualization, Writing—original draft, Writing—review & editing, Supervision, Project administration, Funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zaborniak, I., Chmielarz, P. Polymer-modified regenerated cellulose membranes: following the atom transfer radical polymerization concepts consistent with the principles of green chemistry. Cellulose 30, 1–38 (2023). https://doi.org/10.1007/s10570-022-04880-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-022-04880-4