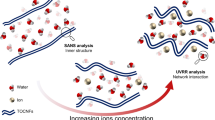
Abstract
Hydrogels formed by cellulose nanofibers (CNFs) find use in a variety of applications. CNF hydrogels generally stiffen and ultimately flocculate with increasing salt concentrations. While charge repulsion explains the behavior of nanocellulose variants that have been stabilized by charged groups, it has been a puzzle why ions have such a pronounced effect also on CNFs with unmodified surfaces. We studied the effect of ionic solutes on native CNF hydrogels, and found that already at very low concentrations of around 1 mM, ions cause crowding of the hydrogels. The ionic solutes used were NaCl, Na2SO4, NaI, NaSCN, and sodium acetate. For the hydrogels, we used low densities of CNFs which lead to relatively weak gels that were highly sensitive to salts. Screening of the electrical double layer could not explain the results at such low ion concentrations. To understand cellulose-ion interactions, we used computational molecular dynamics simulations. The results provide an explanation by the effect of ions on the structure of the hydration layers of the cellulose. Understanding how and why ions affect the properties of native CNF hydrogels can help in for example manufacture of CNFs and when using CNFs as material components, substrates for enzymes, or as rheology modifiers. Ion-effects on the hydration layer of cellulose may also be important for more fundamental understanding of interfacial interactions of cellulose with water under different conditions.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The processing of cellulose to separate it into fibers with small diameters opens possibilities for a wide range of new uses for cellulose. These fibers are often called cellulose nanofibers (CNFs) because their diameters are in the nanometer-range. The CNFs have a largely crystalline packing of long cellulose molecules and have lengths of several micrometers (Pääkkö et al. 2007; Hubbe et al. 2017; Heise et al. 2021; Li et al. 2021b). CNFs therefore have an extremely high aspect ratio, which is the basis of many properties that have practical utilizability. Properties are also affected by the nature of prior chemical processing of the cellulose, leading to variability in content of hemicellulose, possible covalent modifications, or other residues present (Arola et al. 2012).
One property that stands out above others is that CNFs readily form hydrogels (Ajdary et al. 2021). The hydrogel properties dominate the use of CNFs and dictate routes for further processing. Typically, already at solids contents below 1% gels are formed in aqueous media. Because of this high tendency to form gels, high solids contents are difficult to achieve and typical processes give around 5% as the maximal solids content. So-called high consistency processing, can give to up to 20% cellulose, but leads to incomplete fibrillation (Jaiswal et al. 2021). The basis for the pronounced gel-forming ability of CNFs is that it is a colloid dispersion forming a percolated network. The high aspect ratio cellulose fibrils are rigid and form an inherently entangled network structure (Pääkkö et al. 2007). CNF hydrogels are tough and strong compared to polymeric hydrogels at similar concentrations. Because the gelation depends on forming a percolated network of strong and relatively rigid fibrils, the gels are shear thinning and thixotropic, i.e. an applied shear can orient the fibrils leading to a less connected network. CNF gels are not chemically crosslinked which means that the stabilizing fibril contact points can rearrange under deformation forces (Kontturi et al. 2018).
Preparing nanocellulose in other ways that lead to short fibrils of up to a few hundred nanometers result in a low colloidal dispersibility in water and low gelling properties (Fang et al. 2016). Such short, and low aspect-ratio fibrils are called cellulose nano-crystals (CNCs). By modifying CNCs with strongly ionizing groups such as sulphate, a colloidally stabilizing charge repulsion can be obtained leading to a good dispersibility in water (George and Sabapathi 2015; Heise et al. 2021; Li et al. 2021b). By using TEMPO-mediated oxidation of cellulose, yet another type of small-diameter cellulose fibrils can be made. The TEMPO-CNFs have an aspect ratio in between that of CNCs and CNFs, and show enhanced colloidal stability by charge repulsion from the carboxyl groups that are formed on the fibril surfaces during oxidation (Saito et al. 2006).
The hydrogels formed by CNFs have found a wide range of uses. In particular, such hydrogels have turned out to form excellent matrices for growing mammalian cells (Bhattacharya et al. 2012; Lou et al. 2014). Because the CNF network forms a structurally rigid environment with a low solids content, the cells can thrive as nutrients can flow and the CNF network crosslinks do not inflexibly restrict growth while still keeping cells well dispersed. The CNF gels are shear thinning which is useful in the processing and application of the gels. Similar properties lead to uses of CNFs as wound dressings, scaffolds for controlled release of drugs, and immobilization matrices in bioanalytical devices. CNF hydrogels have also been applied as rheology modifiers in paints, cosmetics, and in the food industry (Hubbe et al. 2017; Li et al. 2021b). The biologically neutral properties of cellulose leads to low non-specific binding of biomolecules, low immunological response, and generally low toxicity and good biocompatibility (Lin and Dufresne 2014). In the absence of cellulose-degrading microbes, the CNFs remain chemically and physically highly stable. The very wide range of uses of CNFs in combination with the low environmental burden of both production and final disposal are additional factors making CNF materials attractive for applications (Barhoum et al. 2020).
An understanding of how gels are affected by solution conditions such as ionic solutes is important for many applications. The variants of CNFs that have been chemically modified to have charged surface groups, such as in TEMPO-CNFs, show a decrease of colloidal stability when salts are added (Fukuzumi et al. 2014). The widely known Derjaguin–Landau–Verwey–Overbeek (DLVO) theory predicts that at low ionic strength the electric double layer repulsion keeps the cellulose fibrils separated. At increased ionic strength, the repulsion is screened, with the screening measured by the decrease of Debye screening length. With the resulting decreasing extent of electrostatic forces, van der Waals attractions become dominant leading to aggregation and stronger networks. Experimental observations for TEMPO-CNFs and other charged variants are in line with these predictions (Nordenström et al. 2017; Hubbe et al. 2017; Mendoza et al. 2018). Because the native cellulose does not naturally have ionizable groups, it would follow that CNFs obtained by direct fibrillation of cellulose should be insensitive to increased salt concentrations. This is however not what is observed. At increased salt concentrations, gel stiffening and flocculation occurs even for unmodified CNF gels. Sim et al. showed that the increasing amount of salt in the cellulose hydrogel system (10 mM to 3 M NaCl) induced flocculation and higher water drainage during drying (Sim et al. 2015). Saarikoski et al. came to the a similar conclusion showing in their study that NaCl concentrations of 0.1 and 1 mM did not show significant effects on the rheology of unmodified CNF hydrogels but addition of 10 mM and more caused significant flocculation (Saarikoski et al. 2012). Studying divalent cations, Rahmini et al. observed that gel strength increases with higher concentrations of ions (Rahmini et al. 2020). These results together show clearly that the use of unmodified CNFs is strongly affected by the presence of ionic cosolutes, thus demonstrating the need for a deeper understanding of underlying mechanisms. One such reason for this unexpected salt-dependency of the gelling properties has been suggested to relate to residual sugar acids present in the CNFs stemming from remaining hemicellulose (Li et al. 2021a).
To gain further understanding of the unexpected salt dependency of the rheological properties of unmodified CNFs, we here studied the effects of different salt ions at concentrations between 0.25 and 50 mM by conducting small deformation oscillatory rheology experiments. In order to explain our experimental results, we used molecular modelling together with assessment of expected fibril-fibril separations to assess the interactions of salt ions with the fibril surface and to aid in interpretation of the data.
Materials and methods
CNFs were prepared according to earlier work (Skogberg et al. 2017). Briefly, never-dried bleached Kraft birch pulp was disintegrated with an ultra-fine friction grinder (Masuko Supermasscolloider) with rotation speed of 1500 rpm followed by ten passes through a high-pressure fluidizer (Microfluidics). The resulting samples with a solid content of 1.69% (w/v) were then stored at 4 °C.
Reagents. All salts were of analytical grade and purchased from Sigma.
Sample preparation A CNF stock solution of 0.82% (w/v) was prepared in MQ water and 1.446 mL of the stock was homogenized by tip sonication on ice for 4 min (2 s on/off cycles) with 20% amplitude (QSonica Q500). After sonication, 1.172 mL of CNF stock solution was taken separately and diluted with different salt stock solutions (0.426 mL) and MQ water (344 mL) to yield a final CNF concentration of 0.6% (w/v) CNFs and salt between 0 and 50 mM. The dispersion was mixed for 5 s using a lab-bench vortex. The pH of the solutions was measured to verify the effect of each salt on the CNF solution pH. The pH varied between 6.7 and 7.3 for samples with 0.25–2 mM of salt. The rheology experiments were carried out always 25 min after sample preparation.
Rheological measurements were carried out with an Anton Paar MCR302 strain-controlled Rheometer (Anton Paar GmbH) using a cross-hatched 25 mm parallel plate geometry and a Peltier element. First, a time sweep with constant strain (1%) and constant frequency (0.628 rad/s) was done to equilibrate the sample. The frequency sweep with a constant strain (1% strain) was measured from 0.1 to 628 rad/s angular frequency. The strain sweep with a constant angular frequency (0.628 rad/s) was measured from 0.01 to 100% to ensure covering the linear visco-elastic region (LVR) of each sample.
Crowding points were extracted based on continuous curves obtained by cubic spline fitting by Matlab to the measured Tanδ values vs salt molarity. To enhance the stability of the spline fit in the few to tens of mM measurement range, between the 20.0 mM and 50 mM measured data points, spline fit stability points were added at 2 mM intervals positioned linearly based on the 20.0 mM and 50 mM measured values. The actual cubic spline interpolation was done using a logarithmic axis for the salt molarity and a linear axis for the Tanδ values.
The all-atom molecular dynamics simulations employ GROMACS-2016.6 and GROMACS-2019.3 software (Berendsen et al. 1995; Spoel et al. 2005; Páll et al. 2014; Abraham et al. 2015). The glucose chains of cellulose are described by the carbohydrate parameters of the CHARMM36 force field (Vanommeslaeghe et al. 2010; Guvench et al. 2011). The explicit water was described by the TIP3P water model (Jorgensen et al. 1983). Na+ and Cl− ions follow the CHARMM forcefield.
For the simulations, a cellulose 1ß crystal structure was constructed using Cellulose Builder (Gomes and Skaf 2012). The modelled crystal consisted of six glucose chain layers containing six glucose chains of 10 glucose units each. Periodic boundary conditions were implemented such that the glucose chains spanned the simulation box as infinite chains. The layers were oriented such that they formed a slab with uniform {100} cleavage plane ({− 100} plane at opposite facet of the crystal) in the simulations box. The cleavage plane exposes a layer of origin chains of cellulose. The periodic simulation box followed crystal symmetries and had initially box vector lengths of 5.2 nm, 4.9 nm, and 14.0 nm, see Fig. 1.
Temperature in the simulations was controlled by the stochastic V-rescale thermostat (τ = 0.2 ps) with 300 K reference temperature while anisotropic Parrinello-Rahman coupling (τp = 2.0 ps) with 1 bar reference pressure is used for pressure control (Parrinello and Rahman 1981; Bussi et al. 2007). Long range electrostatics employed the smooth Particle-Mesh Ewald (PME) algorithm with 4th order cubic interpolation and 0.16 nm grid spacing for the FFT (Essmann et al. 1995). The short-range electrostatics and Lennard–Jones truncation distance was 1.0 nm. Dispersion corrections for energy and pressure were applied. A time step of 2 fs and the leap-frog integrator was used for the simulations.
After solvation of the glucose chain slab, 0.1 M or 1.0 M Na+ and Cl− as ions were introduced to the simulation system by Gromacs tools with the purpose of generating insight on how the added salt ions influence molecular level interactions of the cellulose fibrils. Of the possible ion binding sites at the {100} cellulose surface, the easily accessible O6 oxygen and its positively charged hydrogen HO6 were selected as the reference Na+ and Cl− binding sites, see Fig. 1. The ion concentration in the modelling significantly exceeds the experimental concentration because the resulting 22 or 215 Na+ and Cl− ions in the simulation volume leads to better statistical sampling. After energy minimization, a 1 ns pre-optimization of the glucose chain slab in NPT ensemble was performed. Following this, to simplify structural analysis when assessing the ion and water interactions, the C1 and C4 atoms of each glucose chain were constrained by a harmonic force (force constant 1000 kJ/(mol nm2), see Fig. 1. This constrains the cellulose crystal but enables water and ions relax freely around it. The production run was 70 ns in the NPT ensemble out of which, the initial 30 ns was disregarded as relaxation period. In analyzing ion binding to fibril surface, a cut-off distance Rcut = 0.57 nm between the ion and the oppositely charged surface atom was used to determine ion binding. The value was chosen to cover ion binding with full hydration shell at all possible binding sites based on the radial distribution function minimum after the hydrated binding peak. VMD was used for molecular visualizations (Humphrey et al. 1996).
Fibril mean separation distances as the function of fibril length were estimated by a self-implemented Matlab script. The fibrils were described as cylindrical rods of 4 nm diameter varying length between 200 nm and 1 µm. For the estimate, 803–1508 rods were placed in a volume of 0.53–3.44 μm3. The rods were placed in the volume with their centers of mass at random positions in the volume with also rod orientations randomized. A 4 nm diameter, estimated based on fibril diameter, was used as a lower separation bound, i.e. rod overlap was not permitted. Because the randomization of rod orientations leads to sparser rod concentration near the box boundaries, actual analysis was done with 247–506 rods box volumes of 0.22–1.0 μm3 in the center of the calculation volume. The reported quantity is an average of 8 samplings. The standard deviation was used as the error estimate.
Results and discussion
Initially we used small deformation oscillatory rheology to analyze a 0.6% (w/v) CNF dispersion in deionized water (Fig. 2a). The dispersion behaved as a weak percolating gel network with solid-like behaviour at low frequency and a crossover of moduli (G’ = G”) at ~ 103 rad/s (Rosén et al. 2021). The CNF dispersion in water had a positive slope of G’ with increasing frequency.
Angular frequency sweep at 1% amplitude strain for 0.6% (w/v) CNF network with increasing amount of NaCl. a 0.6% (w/v) CNFs in pure water showing a crossover of G’ and G” at 103 rad/s. b 0.6% (w/v) CNFs with 0.25 to 50 mM NaCl. The crossover points of G’ and G” are indicated by circles. G’ is shown as a solid line and G” as dashed a line
As a next step we increased the concentration of NaCl in the solution. We noted that at already 0.25 mM NaCl the network behavior changed, with an increase in both G’ and G” (Fig. 2b). In contrast to the behavior of CNFs in water, G’ was unvarying over a more extended range at low angular frequencies. Overall, higher concentrations of NaCl resulted in increasing values of G’, suggesting that NaCl led to a more highly connected percolating network (Pääkkö et al. 2007). However, the increase of G’ reached a maximum at around 2 mM, whereafter differences were minor. This indicates that under 2 mM NaCl, the percolating system had not reached a saturating degree of bridging, and after 2 mM NaCl the bridging became saturating (Otsubo 1990).
At low NaCl concentrations, between 0.25 and 1 mM, the crossover of G’ and G” appeared earlier (below 100 rad/s than for CNFs in only water. On the other hand, for samples with over 1 mM NaCl, the crossover point increased to above 100 rad/s (Fig. 2b). The crossover point is inversely proportional to the relaxation time of the system as shown in Eq. 1 (Miri 2010). The relaxation time can be described as the time required for the sample to relax after removal of an external applied stress.
Therefore, the higher the frequency at which the crossover appears, the shorter is the relaxation time. The systems with low NaCl concentration (0.25–1 mM), relax more slowly than CNFs in pure water, which means that these systems need more time to recover from applied stress. The CNF hydrogels with above 2 mM NaCl have a shorter relaxation time compared to CNFs in pure water, and their network connections are able to recover more quickly.
Amplitude strain sweeps (Fig. 3) show a linear viscoelastic region (LVR) that extends beyond 1% amplitude strain for all samples. Samples containing low concentrations of NaCl (0.25–1 mM) could withstand more strain before the yield point than the CNF network without any salt (Fig. 3a and SI Fig. 1). We also note that when more than 1 mM NaCl was added, the yield point occurred at lower amplitude strains and the LVR was shorter (Fig. 3a and SI Fig. 1). Similarly, as in the frequency sweeps, adding NaCl above 1–2 mM did not lead to a further increase in moduli. The data therefore suggest that a small addition of NaCl (0.25–1 mM) led to a more connected CNF system than that formed in only water. This resulted in an ability for the network to withstand increased deformations. At NaCl concentrations above 1 mM, the connectivity still increased (higher G’) but their nature seemed to change, as the system failed at lower strains. The response indicates more connections, yet a more easily flowing system.
Amplitude strain sweeps for 0.6% (w/v) CNFs with varying concentrations of NaCl. a Storage modulus, G’, of each measured sample. b Loss modulus, G”, for each measured sample. The black circles drawn on 0.25 mM NaCl data and 1 mM NaCl data indicate the rise in G” values prior to collapse of the network
The amplitude strain sweeps further show that with increasing strain, there is a rise in the G” for samples with salt after the onset of non-linear behavior (indicated with circles in Fig. 3b). This indicates a temporary internal structuring that resists deformation and forms prior to collapse of the network. This structuring can be thought as being temporary flock structures within the network, i.e., local points in the system with denser population of connections. Such a rise in G” at the onset of yield, is typical for colloidal systems (Zakani et al. 2018). After the internal structures have broken the structure fails completely and starts to flow. In our system, these internal structures seem to be promoted by the presence of the salt. The rate of reduction in moduli with increasing strain shows some variability that does not correlate with any particular change in experimental parameters. This variability between samples probably reflects the sensitivity of the kinetic mechanisms of floc formation and the CNF network collapse. Therefore, the behavior at high strains were not used to draw conclusions about the systems, but are shown in order to convey a more complete impression of the data.
To further analyze the effect of salt concentration on CNF hydrogel network structure we next plotted the Tanδ values (G”/G’) against changing NaCl concentration for each measured angular frequency (Fig. 4). Cubic spline fitting was used to connect data from experiments at different salt concentrations. We could identify a transition of the system from gel state to strong gel state, i.e. a crowding point induced by added NaCl and seen as a crossover of the Tanδ curves (Arola et al. 2018). Above the crowding point the hydrogel turns into a strong and arrested hydrogel. This crowding point occurred close to 2 mM of NaCl and was seen only at the lowest end of the frequency range (Otsubo 1990; Arola et al. 2018).
Tanδ versus NaCl concentration for measured frequencies. a Graph showing the whole range of measured frequencies. Local maxima (tip-up triangles) and local minima (top-down triangles) are indicated on the curves. b Graph showing the crossover of Tanδ at lowest range of frequencies indicating a crowding point at slightly below 2 mM of NaCl
We next explored the system with a series of different types of salts. The salts were: Na2SO4, NaI, NaSCN, and sodium acetate (NaOAc). These were chosen to span the anionic part of the Hofmeister ranking series for salts (Kang et al. 2020). Very similar frequency and strain behavior was noted regardless of the salt used (SI Figs. 2, 3). Analysing Tanδ, a very similar crowding behavior with crossover points at closely the same concentration (1–2 mM) was noted for all data sets (Fig. 5). i.e., there was no correlation between our results and the Hofmeister ranking.
We next consider theoretical underpinnings for our observations. The salt concentrations at which the transitions in the study occur, are very low, between 0.5 and 2 mM. Electrostatic screening in solution can be described by the Debye screening length (Eq. 2).
In this equation, \(\varepsilon_{0}\) is the permittivity of vacuum, \(\varepsilon_{r}\) the relative permittivity, \(k_{{\text{B}}}\) the Boltzmann constant, T temperature, \(N_{A}\) Avogadro’s constant, q the elementary charge, and I the ionic strength of the solution. In practice, the Debye screening length measures the distance at which the magnitude of electrostatic interactions induced by charges in solutions decreases by a factor 1/e, where e is Euler’s number. At 25 °C it is around 3 nm for a 10 mM monovalent salt solution and 9.6 nm for a 1 mM solution. This means that Coulombic interactions between charged groups or ions in the solution can be considered to remain unscreened at distances up to 3–10 nm at the salt concentrations in which the Tanδ crossover phenomena appear. As the observed Tanδ crossover appears in such low salt concentrations that the electrostatic interactions still remain relatively unscreened, we conclude that merely the possible presence of ionized groups and their charge distribution at the cellulose surface does not provide an explanation for our observations.
To build a hypothesis for the mechanism, we turned to molecular modelling in atomistic detail. For this purpose, we focus here on the cellulose crystal {100} surface in terms of ion binding and water interactions. The surface of cellulose has several potential binding sites for both Na+ and Cl− ions. Analysis of the MD trajectories reveals that Na+ and Cl− ions bind very weakly to the surface, with interactions strengths comparable to the thermal energy. At 0.1 M NaCl, about 2–3% of possible exposed polar oxygens (Na+) or hydrogens (Cl−) have at any given time an ion closer than Rcut = 0.57 nm. Both Na+ and Cl− bind to a similar, low degree, thus not resulting in a net charge accumulation. Notably, in the concentrations studied experimentally in this work, the occupancy fraction can be expected to be very low based on these modelling results. This low degree of ion binding, together with the lack of effect of changing the specific anion species (Fig. 5) leads us to look in detail at the water interactions and the effect of ions on those.
Our simulations showed that, in the absence of NaCl, the ordering of water is affected by the cellulose surface to approximately 1 nm distance from the surface (Fig. 6a), which is consistent with previous works (Heiner and Teleman 1997; Heiner et al. 1998; Hakalahti et al. 2017; Mudedla et al. 2021). The addition of NaCl does not significantly influence this ordering of water, as seen in the comparison of water density curves in Fig. 6a, which is consistent with the low number of ions bound. However, the presence of added salt nevertheless can be seen in the corresponding dipole moment orientation peaks (Fig. 6b). As the presented data are averaged, the observed difference indicates that salt has strong effect on the ordering of water molecules locally at the level of dipole moments. The significance of this is that water becomes more ordered at localized sites when salt is present, even though the mean density of water molecules did not change.
Molecular level water ordering at the cellulose fibril surface and around the ions based on atomistic detail molecular dynamics simulations. a Presents water density variations near cellulose crystal {100} surface at different salt concentrations and b the corresponding z-axial orientational ordering of water molecules. The angle θz is the angle between z-axis and the dipole moment of water, see cartoon. c The water mass density around Na+ and Cl.− ions in bulk solution and near the cellulose fibril. d Visualizes the distance calculation setup. Note that the mass density graphs show water ordering via water oxygen and hydrogen mass difference
For the point of building a hypothesis and to obtain a qualitative understanding on how the ions influence the cellulose surface, we selected for further examination the negatively charged and easily accessible O6 oxygen as a Na+ binding site, and its positively charged hydrogen HO6 as the Cl− binding reference site, see Figs. 1 and 6d. In our simulation model, Na+ binds preferentially at z-axial distance of 0.46 nm and Cl− at 0.41 nm distance from the reference site. Figure 6c shows that water around both the Na+ and Cl− ions orients very strongly with the ordering extending to around 0.55–0.6 nm distance from the ion center. Around the ions, the first hydration layer is strongly oriented while the orientation of the second hydration shell is weaker and more extended, as expected. Comparison of panels A and C in Fig. 6 shows that the ions interacting with the cellulose surface oppositely charged receptor sites penetrate the ordered water layer of the cellulose fibril surface. However, the data from simulations show only minor differences in the water density around the ions for bulk solvated ions versus those at the interface at a binding site. Importantly, this means that the salt ions do not lose or reorient their hydration shells significantly upon binding to cellulose. Instead, the water ordering is combined with the hydration layer of the cellulose surface, restructuring the water at the surface and increasing the amount of ordered water. This is shown in Fig. 6b in the change in the dipole moment orientation distribution with added ions. However, a simple consideration of the widths of the hydration shell reveals that the added ions do not significantly expand the ordered water layer in the direction perpendicular to the surface, i.e. in the z-direction. However, at the locations of the ions, the degree of water ordering remains strong to the full hydration layer distance, i.e. to a much larger distance from the fibril surface in the z-direction than in the absence of ions. Enhanced ordering in the interfacial water extends to about 1.2 nm from the surface in the z-direction when ions are at the interface. Approximately 1 nm of this ordered water is strongly ordered (main water ordering peaks in Fig. 6).
Data obtained from the simulations show in a qualitative way how the ionic species affects the ordering of water around the cellulose surface, but the simulations are not applicable for assessing the ion binding fraction at experimentally examined concentrations. The higher salt concentrations used in modelling (0.1 M and 1 M NaCl) than in experiments lead to a larger fraction of the CNC fibril surface being influenced by the ions in modelling, and consequently, enhanced peak height differences in Fig. 6. The higher concentrations were used because they allowed improved statistical sampling in the simulations. A possible drawback of the elevated electrolyte concentrations could be the appearances of nonidealities in ion solvation and ion pairing in the modelling. However, since both examined concentrations lead to the same response in terms of peak positions, peak widths, and binding sites, we conclude that nonidealities in ion solvation did not appear in the simulations at such degree that they would qualitatively change the outcome.
To assess the effect of the increased structural order of water near the cellulose surface we next constructed a model to estimate the frequency of occurrence of close contacts between fibrils in a CNF dispersion. Figure 7 shows the mean distance and the distance distribution calculated for randomly distributed fibrils of length varying between 200 nm and 1 µm in a 0.6% (w/v) cellulose fibril solution. For this estimate, the fibrils were considered to be rigid, cylindrical rods of 4 nm in diameter, randomly distributed in solution. In Fig. 7, a distribution plot of interfibrillar distances is shown for fibrils of different length. The length of the fibrils of the CNFs used in the rheology experiments was well over 1 µm. For fibrils over 1 µm in length, we estimate from the calculations that over 2/3 of the fibrils have contact points with separations less than 2.4 nm. This means that the properties of the gel network can easily be perturbed through affecting the nature of these contact points. Additionally, the slope of the curve at around 2 nm is relatively steep which means that should the distance at which the fibrils form contacts increase, the number of contact points is affected significantly. For example, going from 2 to 2.4 nm, the number of contacts increases 10% for 1 µm long rods and almost 20% for 200 nm long rods.
Estimate of minimum distance R distribution for cellulose fibrils approximated as randomly distributed cylinders 4 nm in their diameter and varying length L at 0.6% (w/v) concentration. a The R distributions for fibril lengths of 200 nm, 600 nm and 1 μm. The magenta region and percentage indicate the fraction of fibrils within 2 nm distance from each other. The solid line presents a Gaussian fit to the distribution. Data for intermediate lengths provided in Supporting Information Figure S4. b Scheme showing the parameters used in the calculations
The data above allows us to build a more complete picture of underlying mechanisms. Firstly, the existence of two regimes with a critical point at around 2 mM of salt is evident throughout the rheology data. Comparisons of Tanδ at different frequencies tells us that the observed critical point is a crowding point (Otsubo 1990; Arola et al. 2018). More generally, crowding points often indicate liquid-to-gel transitions and indicate a critical gelling concentration (cg) for a given system, for example brought forward by an additive causing crosslinking (Otsubo 1990). In liquid-to-gel transitions, the Tanδ-values are independent of the measurement frequency for a large range of frequencies (Chambon and Winter 1985; Winter and Chambon 1986). However, in this study, the system was a gel already prior to salt addition and the Tanδ-values were independent of the measurement frequency only for relatively low frequencies. This means that the additives did not cause a gelling of the system but rather a transition in the gel behavior, such as a transition from a gel to a more arrested and stronger gel (Arola et al. 2018).
Considering both the Tanδ response, the insensitivity of the response to salt species and the modelling considerations, we conclude that the observed critical point and the transition from a gel to a stiff gel for the material is caused by the salt ions causing bridging of cellulose nanofibrils. This occurs dominantly via water interactions, i.e. by (1) perturbing the ~ 1 nm ordered surface water layer of the fibrils and (2) by ordering the water layer strongly, causing local points of stronger orientation of water in the layer and increase in the ordered water layer distance at the local points. To a lesser degree, also (3) the increase in water ordering and possible changes in the distance extent of oriented water from the cellulose surface contribute. Altogether, these allow more and stronger contacts to form between fibrils.
The proposed salt-cellulose interaction helps to understand the generally variable results experienced in studying the hydrogel properties of dispersions of fibrillated cellulose. This is because trace amounts of salts are easily present, or are introduced in an uncontrolled manner in different treatments of the cellulose in experimental setups, making it difficult to draw conclusions on the exact reasons for observed effects. More widely we suggest that the effect of ions on the hydration layer can be of importance in the different uses of nanocellulose and their assembly processes, and that this experimental parameter should be carefully considered both in practical applications of cellulose hydrogels and in research using hydrogel properties to draw conclusions of experimental results. Many hydrogel applications of CNFs involve low ionic strength formulations or intermediate preparations with low ionic strength. The high sensitivity already at very low salt concentrations shows that this parameter must be carefully controlled in any preparation of CNF hydrogels as key rheological parameters are strongly affected. The suggested mechanism for the effect of salt is inherent to the structure of cellulose, and forms a key parameter for its use.
References
Abraham MJ, Murtola T, Schulz R et al (2015) GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2:19–25. https://doi.org/10.1016/j.softx.2015.06.001
Ajdary R, Tardy BL, Mattos BD et al (2021) Plant nanomaterials and inspiration from nature: water interactions and hierarchically structured hydrogels. Adv Mater 33:2001085. https://doi.org/10.1002/adma.202001085
Arola S, Malho J-M, Laaksonen P et al (2012) The role of hemicellulose in nanofibrillated cellulose networks. Soft Matter 9:1319–1326. https://doi.org/10.1039/C2SM26932E
Arola S, Ansari M, Oksanen A et al (2018) The sol–gel transition of ultra-low solid content TEMPO-cellulose nanofibril/mixed-linkage β-glucan bionanocomposite gels. Soft Matter 14:9393–9401. https://doi.org/10.1039/C8SM01878B
Barhoum A, Jeevanandam J, Rastogi A et al (2020) Plant celluloses, hemicelluloses, lignins, and volatile oils for the synthesis of nanoparticles and nanostructured materials. Nanoscale 12:22845–22890. https://doi.org/10.1039/D0NR04795C
Berendsen HJC, van der Spoel D, van Drunen R (1995) GROMACS: a message-passing parallel molecular dynamics implementation. Comput Phys Commun 91:43–56. https://doi.org/10.1016/0010-4655(95)00042-E
Bhattacharya M, Malinen MM, Lauren P et al (2012) Nanofibrillar cellulose hydrogel promotes three-dimensional liver cell culture. J Control Release 164:291–298. https://doi.org/10.1016/j.jconrel.2012.06.039
Bussi G, Donadio D, Parrinello M (2007) Canonical sampling through velocity rescaling. J Chem Phys 126:014101. https://doi.org/10.1063/1.2408420
Chambon F, Winter HH (1985) Stopping of crosslinking reaction in a PDMS polymer at the gel point. Polym Bull 13:499–503. https://doi.org/10.1007/BF00263470
Essmann U, Perera L, Berkowitz ML et al (1995) A smooth particle mesh Ewald method. J Chem Phys 103:8577–8593. https://doi.org/10.1063/1.470117
Fang W, Arola S, Malho J-M et al (2016) Noncovalent dispersion and functionalization of cellulose nanocrystals with proteins and polysaccharides. Biomacromolecules 17:1458–1465. https://doi.org/10.1021/acs.biomac.6b00067
Fukuzumi H, Tanaka R, Saito T, Isogai A (2014) Dispersion stability and aggregation behavior of TEMPO-oxidized cellulose nanofibrils in water as a function of salt addition. Cellulose 21:1553–1559. https://doi.org/10.1007/s10570-014-0180-z
George J, Sabapathi S (2015) Cellulose nanocrystals: synthesis, functional properties, and applications. Nanotechnol Sci Appl 8:45–54. https://doi.org/10.2147/NSA.S64386
Gomes TCF, Skaf MS (2012) Cellulose-builder: a toolkit for building crystalline structures of cellulose. J Comput Chem 33:1338–1346. https://doi.org/10.1002/jcc.22959
Guvench O, Mallajosyula SS, Raman EP et al (2011) CHARMM additive all-atom force field for carbohydrate derivatives and its utility in polysaccharide and carbohydrate-protein modeling. J Chem Theory Comput 7:3162–3180. https://doi.org/10.1021/ct200328p
Hakalahti M, Faustini M, Boissière C et al (2017) Interfacial mechanisms of water vapor sorption into cellulose nanofibril films as revealed by quantitative models. Biomacromolecules 18:2951–2958. https://doi.org/10.1021/acs.biomac.7b00890
Heiner AP, Teleman O (1997) Interface between monoclinic crystalline cellulose and water: breakdown of the odd/even duplicity. Langmuir 13:511–518. https://doi.org/10.1021/la960886d
Heiner AP, Kuutti L, Teleman O (1998) Comparison of the interface between water and four surfaces of native crystalline cellulose by molecular dynamics simulations. Carbohydr Res 306:205–220. https://doi.org/10.1016/S0008-6215(97)10053-2
Heise K, Kontturi E, Allahverdiyeva Y et al (2021) Nanocellulose: recent fundamental advances and emerging biological and biomimicking applications. Adv Mater 33:2004349. https://doi.org/10.1002/adma.202004349
Hubbe MA, Tayeb P, Joyce M et al (2017) Rheology of nanocellulose-rich aqueous suspensions: a review. BioResources 12:9556–9661
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14:33–38. https://doi.org/10.1016/0263-7855(96)00018-5
Jaiswal AK, Kumar V, Khakalo A et al (2021) Rheological behavior of high consistency enzymatically fibrillated cellulose suspensions. Cellulose 28:2087–2104. https://doi.org/10.1007/s10570-021-03688-y
Jorgensen WL, Chandrasekhar J, Madura JD et al (1983) Comparison of simple potential functions for simulating liquid water. J Chem Phys 79:926–935. https://doi.org/10.1063/1.445869
Kang B, Tang H, Zhao Z, Song S (2020) Hofmeister series: insights of ion specificity from amphiphilic assembly and interface property. ACS Omega 5:6229–6239. https://doi.org/10.1021/acsomega.0c00237
Kontturi E, Laaksonen P, Linder MB et al (2018) Advanced materials through assembly of nanocelluloses. Adv Mater 30:1703779. https://doi.org/10.1002/adma.201703779
Li M-C, Wu Q, Moon RJ et al (2021a) Rheological aspects of cellulose nanomaterials: governing factors and emerging applications. Adv Mater 33:2006052. https://doi.org/10.1002/adma.202006052
Li T, Chen C, Brozena AH et al (2021b) Developing fibrillated cellulose as a sustainable technological material. Nature 590:47–56. https://doi.org/10.1038/s41586-020-03167-7
Lin N, Dufresne A (2014) Nanocellulose in biomedicine: current status and future prospect. Eur Polym J 59:302–325. https://doi.org/10.1016/j.eurpolymj.2014.07.025
Lou Y-R, Kanninen L, Kuisma T et al (2014) The use of nanofibrillar cellulose hydrogel as a flexible three-dimensional model to culture human pluripotent stem cells. Stem Cells Dev 23:380–392. https://doi.org/10.1089/scd.2013.0314
Mendoza L, Batchelor W, Tabor RF, Garnier G (2018) Gelation mechanism of cellulose nanofibre gels: a colloids and interfacial perspective. J Colloid Interface Sci 509:39–46. https://doi.org/10.1016/j.jcis.2017.08.101
Miri T (2010) Viscosity and oscillatory rheology. In: Norton IT, Spyropoulos F, Cox P (eds) Practical food rheology. Wiley-Blackwell, Oxford, pp 7–28
Mudedla SK, Vuorte M, Veijola E et al (2021) Effect of oxidation on cellulose and water structure: a molecular dynamics simulation study. Cellulose 28:3917–3933. https://doi.org/10.1007/s10570-021-03751-8
Nordenström M, Fall A, Nyström G, Wågberg L (2017) Formation of colloidal nanocellulose glasses and gels. Langmuir 33:9772–9780. https://doi.org/10.1021/acs.langmuir.7b01832
Otsubo Y (1990) Elastic percolation in suspensions flocculated by polymer bridging. Langmuir 6:114–118. https://doi.org/10.1021/la00091a016
Pääkkö M, Ankerfors M, Kosonen H et al (2007) Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels. Biomacromolecules 8:1934–1941. https://doi.org/10.1021/bm061215p
Páll S, Abraham M, Kutzner C et al (2014) Tackling exascale software challenges in molecular dynamics simulations with GROMACS. EASC. https://doi.org/10.1007/978-3-319-15976-8_1
Parrinello M, Rahman A (1981) Polymorphic transitions in single crystals: a new molecular dynamics method. J Appl Phys 52:7182–7190. https://doi.org/10.1063/1.328693
Rahmini JS, Seong H-A, Shin S-J (2020) Impact of divalent cations on the rheology of cellulose nanofibrils. J Korea TAPPI 52:78–86. https://doi.org/10.7584/JKTAPPI.2020.04.52.2.78
Rosén T, Hsiao BS, Söderberg LD (2021) Elucidating the opportunities and challenges for nanocellulose spinning. Adv Mater 33:2001238. https://doi.org/10.1002/adma.202001238
Saarikoski E, Saarinen T, Salmela J, Seppälä J (2012) Flocculated flow of microfibrillated cellulose water suspensions: an imaging approach for characterisation of rheological behaviour. Cellulose 19:647–659. https://doi.org/10.1007/s10570-012-9661-0
Saito T, Nishiyama Y, Putaux J-L et al (2006) Homogeneous suspensions of individualized microfibrils from TEMPO-catalyzed oxidation of native cellulose. Biomacromolecules 7:1687–1691. https://doi.org/10.1021/bm060154s
Sim K, Lee J, Lee H, Youn HJ (2015) Flocculation behavior of cellulose nanofibrils under different salt conditions and its impact on network strength and dewatering ability. Cellulose 22:3689–3700. https://doi.org/10.1007/s10570-015-0784-y
Skogberg A, Mäki A-J, Mettänen M et al (2017) Cellulose nanofiber alignment using evaporation-induced droplet-casting, and cell alignment on aligned nanocellulose surfaces. Biomacromolecules 18:3936–3953. https://doi.org/10.1021/acs.biomac.7b00963
Spoel DVD, Lindahl E, Hess B et al (2005) GROMACS: fast, flexible, and free. J Comput Chem 26:1701–1718. https://doi.org/10.1002/jcc.20291
Vanommeslaeghe K, Hatcher E, Acharya C et al (2010) CHARMM general force field: a force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J Comput Chem 31:671–690. https://doi.org/10.1002/jcc.21367
Winter HH, Chambon F (1986) Analysis of linear viscoelasticity of a crosslinking polymer at the gel point. J Rheol 30:367–382. https://doi.org/10.1122/1.549853
Zakani B, Ansari M, Grecov D (2018) Dynamic rheological properties of a fumed silica grease. Rheol Acta 57:83–94. https://doi.org/10.1007/s00397-017-1064-6
Acknowledgments
The authors acknowledge that this work was a part of the Academy of Finland’s Flagship Programme under Project Nos. 318890 and 318891 (Competence Center for Materials Bioeconomy, FinnCERES). The work was also supported by Academy of Finland through its Centres of Excellence Programme (2022–2029, LIBER) under Project Nos. 346105 and 346111 and Academy of Finland Projects Nos. 326345, 307474, 311608, 326262, and 309324. Authors also gratefully acknowledge the Bioeconomy Infrastructure and the computational resources provided by CSC—IT Center for Science, Finland, and RAMI—RawMatTERS Finland Infrastructure.
Funding
Open Access funding provided by Aalto University.
Author information
Authors and Affiliations
Contributions
SA supervised the rheological measurements, analyzed the data, conceptualized the work, and wrote the manuscript, KZ performed the modelling and simulations, analyzed the data and wrote relevant parts of the manuscript, BR collected and analyzed data and participated in conceptualizing the work, RV collected and analyzed data and participated in conceptualizing the work, MS supervised the modelling and simulations, analyzed data, conceptualized the work, and wrote the manuscript, MBL supervised and conceptualized the work, and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arola, S., Kou, Z., Rooijakkers, B.J.M. et al. On the mechanism for the highly sensitive response of cellulose nanofiber hydrogels to the presence of ionic solutes. Cellulose 29, 6109–6121 (2022). https://doi.org/10.1007/s10570-022-04664-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-022-04664-w