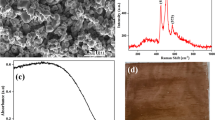
Abstract
Unique technique is currently demonstrated for preparation of ultraviolet protective cotton fabrics with full shielding effect, via self-implantation of palladium (Pd) nanoclusters. Palladium nanoclusters were in-situ immobilized within native and cationized cotton using two different concentrations of palladium precursor (20 and 60 mM) under strong acidic (pH 2) and basic (pH 11.5) media. Cationization (50 and 100%) of cotton fabrics was performed in order to increase the accessibility of fabric for controllable implantation of palladium nanoclusters. Size distribution of palladium nanoclusters in supernatant solution was estimated via Transmission electron microscopy to be 3.2 nm. The estimated data showed that the sample prepared with the highest cationization percent and highest concentration of palladium precursor in strong alkaline medium exhibited the highest yellowness index, color strength and excellent ultraviolet shielding effects. The yellowness index was significantly increased from 15.67 for cationized cotton to 74.99 for the sample prepared with the highest cationization percent and highest concentration of Pd+2 in alkaline medium (Pd-CC (100)4). Tensile strength was insignificantly decreased from 93.2 MPa for cationized cotton to 84.5 MPa for Pd-CC (100)4. Ultraviolet shielding effect was superiorly enhanced with implantation of palladium nanoclusters. The UV protection factor (UPF) was excellency increased from 1.3 (insufficient) for native cotton to 256.6 (excellent) for Pd-CC (100)4. The effect of repetitive washing cycles on the colorimetric data and the results of ultraviolet protection was also studied to affirm the effect of fabric cationization in preparation of highly durable UV-protective fabrics.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increased concern regarding damage resulting from the exposure to microbial organisms, chemical reagents, insecticides, ultraviolet irradiation and pollutants in the last decade, has heightened the requirement for protective clothing materials. Garments today are required to be waterproofing, flame resistant, self-cleaning, pest repellent and microbicidal to protect human body from the infections, ultraviolet irradiation, chemical and biological reagents, be warmer in cold weather and comfortable in summer. Conventional methodologies of finishing application, such as pad-dry-cure or coating that are recently being applied to make the fabrics acquire the microbicidal, ultraviolet shielding, self-cleaning and fire-retardant finishing reagents, are usually combined with increase in fiber thickness, loss of smoothness and drape, lowering the washing fastness, poor of mechanical properties and most importantly reduced the comfortability to the wearers (Durán et al. 2007; Fei et al. 2006; Mehta 2008; Rao et al. 2015; Simončič & Klemenčič, 2016; Vigneshwaran et al. 2006).
The protective clothes could be identified as textile materials that are mainly applicable to protect human body from any external threat. Moreover, there are vital safety issues correlating to their applications as well as the disposal of chemical reagents used for their contemporary finishing. Therefore, the researchers considered with the field of textile industry were continued to look for alternative finishing reagents and technologies that must be environment friendly, with high fastness, low cost and not disadvantageously affect the comfort of clothes while providing efficiency and optimum protection (Baglioni et al. 2005; Hassabo et al. 2019; Subash et al. 2012; Wong et al. 2006; Zhang et al. 2018). Cotton fabrics were exploited in different applications and purposes owing to their outstanding properties like biodegradability, absorbability, softness, and breathability, but disadvantageous with prone to be attacked with microbes especially bacterial strains, poor ultraviolet shielding, and high flammability (Goncalves et al. 2009; Li et al. 2007a, b; Li et al. 2007a, b).
Numerous approaches were considered with investigating different techniques for acquirement different textile materials with number of additional functions, like coloration (Ahmed et al. 2018; Emam & Abdelhameed 2017), self-cleaning (Emam et al. 2018; Emam et al. 2020a, b, c; Rehan et al. 2013), optical activity (Emam et al. 2018), insect repellency (Abdel-Mohdy et al. 2008; Abdelhameed et al. 2017), microbicidal activities (Ahmed et al. 2017a, b; Emam et al. 2020; Ibrahim et al. 2021), electromagnetic interference (EMI) shielding (Gao et al. 2021; Tan et al. 2018; Wang et al. 2021), and ultraviolet shielding (Ates & Unalan 2012; Emam, et al. 2020; Khan et al. 2015; Nazari et al. 2013). In addition to, the protective masking and air-filtering textiles have been prepared for protection from the chemical warfare gases and produced via the immobilization of different functionalizing reagents, like lipophilic activated carbon with efficient adsorption action (X. Li et al. 2011).
Numerous reports were considered with the exploitation of some organic reagents for textiles functionalization, like triclosan for antibacterial potency, benzophenones for ultraviolet shielding, dimethylol dihydroxy ethylene urea for anti-crease performance, fluorocarbons for lipophilic characters, long-chain hydrocarbons and polydimethylsiloxanes for flexibility and softening (Almeida 2006; Hewson 1994). Butane tetra-carboxylic acid, citric acid and maleic acid (Welch 1988; Yang et al. 2010; Yang et al. 1998) were also exfoliated for acquiring cotton fabrics with anti-crease action.
Different types of metallic based nanomaterials were reported to efficiently applicable in various fields (Ali et al. 2021; Karatepe et al. 2021; Tümen et al. 2021), especially sewage treatment, chemical synthesis, hydrogen storage, exhaust gas treatment, and oil refinement (Adams & Chen 2011; Hughes et al. 1995; Lee et al. 2010; Mackus et al. 2015; Meyer et al. 2015; Moon et al. 2014; Zaluski et al. 1995). The priority of nanosized materials is attributed to their high surface area per volume ratio, highly organized composition, high density of coordinative sites, high oxidation activity, and superior mechanical and thermal stabilities (Cano et al. 2017).
Numerous studies were demonstrated textiles functionalization via immobilization of various noble-metal nanostructures such as silver (Ahmed et al. 2018; Emam et al. 2016a, b; Rao et al. 2015; Simončič & Klemenčič, 2016), gold (Hanan B Ahmed et al. 2017a, b; Emam et al. 2017), titanium dioxide (Durán et al. 2007; Fei et al. 2006; Vigneshwaran et al. 2006) and zinc oxide (Baglioni et al. 2005; Hassabo et al. 2019; Zhang et al. 2018). However, palladium (Pd) nanostructures were reported to be efficiently applicable in catalysis (Abdelhameed et al. 2020; Emam & Ahmed 2019; Emam et al. 2020a, b, c; Emam et al. 2020a, b, c; Lim et al. 2010). Palladium was successfully applied as catalyst for facilitating the hydrogen absorptivity and detection owing to its high affinities in absorption of hydrogen (Adams & Chen 2011; Lee et al. 2010; Moon et al. 2014; Rikkinen et al. 2011; Zaluski et al. 1995). Moreover, palladium nanostructures were found to be more efficient in removal of dyes from the aqueous media via the heterogenous catalysis than many typically applied methodologies, like filtration, biological treatment, chemical precipitation, adsorption and techniques. According to our knowledge, no researching studies were considered with exploitation of palladium nanostructures in textile functionalization.
Herein, a novel/investigative approach for preparation of excellent ultraviolet protective cotton fabric is uniquely proposed via self-implantation of palladium nanoclusters. Whereas, for the first time, the immobilized palladium nanoclusters were functionalized in acquirement of the treated fabrics excellent ultraviolet shielding potency. The particle size of the dispersed palladium nanoclusters in supernatant solution was estimated from transmission electron microscopic images. Regulative implantation of palladium nanoclusters was proceeded via immobilization within the polymeric matrix of both native and cationized cotton fabrics. Afterward, the modified fabrics were characterized via infrared spectroscopy (FTIR), scanning electron microscopy (SEM), X-ray diffraction (XRD), colorimetric measurements (color coordinates and color strength), mechanical properties (tensile strength and elongation percentage), and UV-protective action (transmittance percent, ultraviolet protection factor UPF and ultraviolet protection rating).
Experimental work
Materials and chemicals
Palladium chloride (PdCl2, 99%, from Sigma-Aldrich – USA), Sodium hydroxide (99%, from Merck, Darmstadt–Germany), acetic acid and sodium carbonate were of laboratory grade chemicals. 3-Chloro-2-hydroxypropyl trimethyl ammonium chloride (69%) of technical grade chemicals (known as Quatt-188) was purchased under the commercial name CR-2000 from Aldrich. All the chemicals were used as supplied without any further purification. Mill de-sized, scoured and bleached cotton fabric, plain weave, supplied by El-Nasr Company for spinning weaving and Dyeing El-Mahallah El-Kubra, Egypt. The fabric was further purified in the laboratory by washing at 100 °C for 60 min using a solution containing 2 g/L, Na2CO3 and 1 g/L, non-ionic surfactant. Lastly, the fabrics were washed several times with boiling water then with cold water and finally dried at ambient conditions.
Procedure
Cationization of cotton fabrics
Chemical modification of the cotton gauze through cationization was carried out according to literature (Hashem et al. 2009, 2005). The experimental procedure adopted was as follows: 3-Chloro-2-hydroxypropyl trimethyl ammonium chloride (Quat-188) was mixed with sodium hydroxide solution with a molar ratio of 2 (NaOH):1 (Quat-188). The cotton gauze samples were padded in this mixture in two dips and two nips, and then squeezed to a wet pick-up of about 100%. The cotton gauze was dried at 40 °C for 10 min and cured at 120 °C for 3 min. Thus, treated cotton gauze samples were washed with cold water and 1% acetic acid, followed by several washing cycles and finally dried under the normal laboratory conditions.
Self-implantation of palladium nanoclusters
The palladium nanoclusters were self-implanted in to the native and cationized cotton fabrics by dipping method. In this procedure, pieces of cotton fabrics (0.25 g) were impregnated in distilled water and stirred till temperature reached 90 °C then palladium salt solution with specific concentration (20 and 60 mM) was added with liquor ratio of 2:50 at two different pH (2 & 11.5) and left for continuous stirring at 90 °C for 30 min. Afterward, the fabric pieces were air dried before they were instrumentally analyzed. Table 1 represented the samples that were prepared in the current approach under different experimental conditions.
Temperature = 90 ± 3 °C, Time = 30 min, where, 50 and 100 referring to the experimental percent of Quaternary ammonium salt used for cationization.
Characterization and instrumental analysis
Geometrical shape and size distribution of the self-implanted palladium nanoclusters were estimated by using a high-resolution transmission electron microscope (JEOL-JEM-1200; Japan). Size distribution of palladium nanoclusters was evaluated with 4 pi analysis software (from USA) for at least 50 particles. The treated fabrics were characterized via the high-resolution scanning electron microscope (HRSEM Quanta FEG 250 with a field emission gun, FEI Company, Netherlands). Elemental analysis was also estimated using an energy dispersive X-ray analyzer (EDAX AME-TEK analyzer). The infrared spectra of the treated fabrics were obtained by using a Jasco FT/IR 6100 spectrometer. Their spectral mapping data were ranged from 4000 to 400 cm−1 and were determined with 4 cm−1 resolution and 64-time scanning with a rate of 2 mm/sec. Additionally, the prepared fabrics were characterized by powder X-ray diffraction using X’Pert MPD diffractometer system from Philips, at room temperature. Diffraction patterns were estimated in the diffraction angle (2θ) range of 3.5–50° using monochromatized (Cu Kα X-radiation at 40 kV, 50 mA and λ = 1.5406 Å).
The colorimetric data (L, a*, b*, absorbance, color strength [K/S], whiteness index E313 [D65/10] and yellowness index E313 [D65/10] of the treated fabrics were estimated using a spectrophotometer attached with a pulsed xenon lamp (UltraScan Pro, Hunter Lab, USA). The color coordinate parameters of L*, a*, and b* corresponded to the lightness (black/white, 0/100), red/green ratio (+ / −), and yellow/blue ratio (+ / −), respectively (Hanan B Ahmed et al. 2017a, b; Ahmed et al. 2018). The measurement was performed three times for estimation of the average values. The mechanical properties of the treated fabrics were investigated, while, tensile strength (MPa) and elongation at break (%) for the fabrics were estimated related to ASTM method D2256 − 66 T by using the strip test methodology on the tester instrument (Asano machine MFG. Co. Ltd., Japan). Transmission spectral results (T%) for ultraviolet irradiation (UVR) over pristine and cationized cotton before and after the successive implantation of palladium nanoclusters were estimated using JASCO V-750 spectrophotometer (Japan) in the range of 280–400 nm with two nanometers interval. Additionally, ultraviolet protection factor (UPF), resistance in UV-A (315–400) region (UVA) and in UV–B (280–315) region (UVB) were predicted using the AATCC test method 183–2010.44. For each sample, estimation was estimation for two different times, and the average was calculated.
Results and Discussion
Synthesis of palladium nanoclusters
In spite of immobilization of various metallic nanoobjects for textile finishing was extensively studied (Baban et al. 2010; Gorenšek & Recelj 2009; Gulrajani et al. 2008; Ko et al. 2007; Maneerung et al. 2008; Perelshtein et al. 2008; Saengkiettiyut et al. 2008; Wu et al. 2010), but there were no researching approaches that were considered with exploitation of palladium nano-objects for textile finishing. Additionally, no researching reports were studied the direct implantation of palladium nanoclusters within textile matrix. The main challenge of the current approach is to study investigate the superior role of palladium nanoclusters in textile functionalization. Herein, the leverage effect for the direct implantation of palladium nanoclusters on coloration, mechanical properties and UV-protection efficiency of cotton fabrics was systematically studied.
In accordance to literature (H. B. Ahmed et al. 2017a, b; Ahmed et al. 2018; Emam et al. 2016a, b), self-implantation of palladium nanoclusters within cotton fabrics could be hypothesized as the cellulosic building blocks of cotton fabrics with the terminal/alcoholic groups might assist in reduction of palladium ions for ingraining of palladium nanoclusters that could be highly stabilized within the intermolecular spaces of cotton polymeric matrix. Moreover, fabric cationization was performed for cotton before self-implantation of palladium nanoclusters in order to achieve the fulfill goal. Fabric cationization is supposed to enhance the implantation and stabilization of palladium nanoclusters within fabric matrix, while, such chemical modification acquired the fabric more accessible reducing groups to act more actively for ingrowth and stabilization of the requisite nanostructure (Fig. 1).
In more details, according to literature (Fürstner 2008; Kiwi & Graetzel 1979) that were considered with stabilization of metal nanoparticles by ionic liquids, it could be depicted that, cationization of fabrics with quaternary ammonium salts acted in higher efficiency for stabilization of nanoclusters via the electrochemical and steric conceptions, as halogen ions were supposed to be very strongly adsorb on the surface of palladium nanoclusters, while, the surrounding quaternary alkylammonium cations were suggested to constrain close contact between the nanoclusters.
TEM and size distribution
The geometrical features and topography of the implanted palladium nanoclusters that were stably dispersed within the supernatant solution containing the treated fabrics were shown in transmission electron microscopic images (Fig. 2) from which the size distribution (Supplementary file, Fig. S1) was estimated. The microscopic images displayed the successful implantation of palladium nanoclusters with size distribution of 41.3 ± 10.1 nm in alkaline medium (pH of 11.5) in the supernatant solution of native cotton treated with 20 mM of palladium chloride. Quite smaller sized palladium nanoclusters (3.2 ± 0.6 nm) were ingrained and well dispersed within the supernatant solution of cationized fabric treated with palladium precursor under the same experimental conditions, reflecting the pre-eminent effect of cationization in enhancement the accessibility of fabric/cellulosic backbone in the ingrowth of palladium nanoclusters. While, duplication of the percentage of quaternary ammonium salt exploited for cationization from 50 to 100% is insignificantly affected on particle size (4.6 ± 1.7 nm).
SEM
The topography of the surface of the pristine and cationized fabrics before and after implantation of palladium nanoclusters. In order to show the effects of palladium precursor concentration and cationization on the successive implantation of palladium nanoclusters, scanning electron microscopic images (SEM images), energy dispersive x-ray (EDX signals), and the elemental analysis (supplementary file, Fig. S2) of cationized cotton, Pd-C4, Pd-CC(50)4, Pd-CC(100)3 and Pd-CC(100)4 were plotted in Fig. 3. Before successive implantation of palladium nanoclusters, the surface of the cationized fabric seemed to be smoothy, and the specialized signals of carbon, oxygen, nitrogen and chlorine were obviously detected (Fig. 3a). After implantation of palladium nanoclusters, the nanoclusters were clearly dispersed on the surface of the modified fabrics. Whereas, from EDX data, the characteristic peaks of carbon, oxygen, nitrogen, chlorine, and palladium were significantly shown, that affirmed the successive implantation of nanoclusters within the fabrics (Fig. 3b). Moreover, cationization were shown to extensively enhance the implantation of palladium nanoclusters to be regulatory uploaded on the fabric surface as shown in Fig. 3b&c, while, duplication of the cationization percent from 50% (Fig. 3c) up to 100% (Fig. 3e) resulted in more accessibility for implanting of more condensed nanoclusters, which could confirm the postulated mechanism of palladium nanoclusters implantation. By comparing between the microscopic images of the modified fabrics after implantation of palladium nanoclusters (Fig. 3d & e), it is obviously shown that, increment of the concentration of palladium precursor resulted in more dense masses of nanoclusters on the surface of the fabric.
FTIR
The chemical compositions of quaternary ammonium salt, pristine cotton, cationized cotton and fabrics immobilized with palladium nanoclusters were investigated via FTIR (Fig. 4). From the spectral data it could be depicted that the quaternary ammonium salt, pristine and cationized cotton were characterized with absorption signals of O–H (3330–3289 cm−1) and aliphatic C–H (2888 cm−1) and weak bands of C=O (1620 cm−1), asymmetric C–O–C (1472 cm−1), and C–C (1072 cm−1) (Emam & Abdelhameed 2017; Emam & Bechtold 2015). After implantation of palladium nanoclusters, all of the absorbance bands were retained and new band of O–Pd (833 cm−1) was obviously detected, that affirmed the cooperation of the fabric functional groups in stabilization of palladium nanoclusters. Additionally, the absorption bands of C=O (1620 cm−1), H–C–H, and asymmetric C–O–C became more intense, that also affirmed the role of the fabric accessible groups in reduction and stabilization of palladium ions for successive implantation of palladium nanoclusters.
XRD patterns
The aforementioned illustration affirmed the successful implantation of palladium nanoclusters within the fabric matrix through chemical bonding. Figure 5 is plotted for XRD data of cotton and cationized cotton before and after implantation of palladium nanoclusters, and from the represented data it could be declared that, cotton was detected with three characteristic bands at 2θ = 14.9°, 16.7° and 21.5° corresponding to the crystalline structure of cellulose. While, after immobilization of palladium nanoclusters, two characteristic peaks for palladium Pd (111) and Pd (200) were detected at 2θ = 31.8° and 45.4°, respectively, for face centered crystalline Pd (JCPDS data number 89–4897 card) (Emam & Ahmed 2019; Emam, et al. 2020a, 2020b, 2020c; P. Liu et al. 2016a, 2016b; Liu et al. 2016a, 2016b). Moreover, for cationized cotton modified with palladium nanoclusters, an additional peak for Pd (202) at 2θ = 75.4° was appeared, to affirm the effect of cationization in increment the accessibility of fabric for regulative implantation for more of palladium nanoclusters.
Colorimetric data and mechanical properties
From the visual observation of the samples imaged in Fig. 6 it could be noted that, treatment of fabrics with the demonstrated technique resulted in fabric coloration, while, the color was changed from white for native cotton to creamy white with cationization, and under different experimental conditions, the fabric color was developed to yellowish, brownish and lastly to dark black color. The color data of the pristine cotton and cationized cotton after implantation of palladium nanoclusters are presented in Table 2 and supplementary file (Fig. S3). From the plotted data in Table 2, Yellowness index (YI) of the cationized cotton was extremely higher than that of pristine cotton fabrics. For fabrics prepared under alkaline condition, with increment of the concentration of palladium precursor and with duplication of the cationization percentage up to 100%, the lightness (L*) and whiteness index (WI) were significantly decreased, while, color strength (K/S), darkness (b*) and yellowness index were significantly increased.
From the plotted results in Fig. 6, it could be observably shown that, cationized fabrics were exhibited with higher absorbance and color strength values. Additionally, absorbance and color strength were extensively higher for fabrics prepared under alkaline conditions rather than that prepared under acidic pH with increment of palladium salt concentration and duplication of cationization percentage from 50% up to 100%. So, it could be summarized that, yellowness degree, absorbance and color strength could be ordered as follows: Pd-CC (100)4 > Pd-CC (100)3 > > Pd-CC (50)4 > Pd-CC (50)3 ≥ Pd-C4 > Pd-C3 > > cationized cotton > cotton.
The effects of palladium nanoclusters implantation within the polymeric matrix for both of pristine and cationized cotton on the mechanical properties were monitored via estimation of tensile strength and elongation percentage and the results were tabulated in Table 3. Cationization of fabrics and immobilization of palladium nanoclusters resulted in decrement in the tensile strength and elongation percentage. Retention of Elongation percentage and slight diminishing in tensile strength were observed with duplication of cationization percentage. Cationized fabrics immobilized with palladium nanoclusters under alkaline conditions were characterized with lower tensile strength and elongation percentage than that prepared under acidic conditions. Increment of palladium concentration from 20 up to 60 mM resulted in nonsignificant decrement in tensile strength and retention of elongation. This could be logically attributed to that, the chemical medication and immobilization of palladium nanoclusters within the intermolecular spaces of fabric polymeric matrix, resulting in bond breaking within the polymeric chains. However, the decrement in tensile strength for the fabrics after implantation of palladium nanoclusters could be acceptable for application as clothing materials.
UV protection
Cotton based textile materials were widely applicable in various purposes owing to their outstanding characters like biodegradability, softness, and breathability, but they are disadvantageous with prone to microbial attacking, low capability in protection from ultraviolet irradiation, and high flammability (Goncalves et al. 2009; Q. Li et al. 2007a, 2007b; Y. Li et al. 2007a, 2007b). It was recently reported that special metallic oxides like, titanium dioxide, silicon dioxide, zirconium dioxide, magnesium dioxide and zinc oxide, in addition to some of polymeric materials were successfully applicable for coating of cotton fabrics to acquire the treated fabrics characteristic functional properties (Charpentier et al. 2012; Gandhi et al. 2010; Hojjati et al. 2007).
Ultraviolet protection factor (UPF, Table 4) and transmission percent (T%, supplementary file, Fig. S4) could be ascribed as key factors for evaluation of the ultraviolet protection action for all the prepared samples. Ultraviolet shielding property was analyzed within wavelength range of 280 − 400 nm, as shown in supplementary file (Fig. S4). Transmittance percent of cotton fabric is ca. 66% and 83.3% for both UV-A and UV-B, respectively. The blocking percent of the treated fabrics indicated with minor transmittance of UV radiation compared to untreated ones (UVA blocking percent 34.0% & UVB blocking16.7%), i.e., T% of all the prepared samples was observably diminished after implantation of palladium nanoclusters compared to that of the untreated fabric (UVA T% 66.0 & UVB T% 83.3). The blocking percentage of treated cotton fabrics showed that the percent of blocking for UV-B radiation was higher than that of UV-A for all samples prepared from native cotton, in contrast to that prepared from cationized cotton (Table 4). The increment of concentration of palladium precursor (UVB T% for Pd-C3 was 1.6%, while, for Pd-C4 was 1.0%) and processing the palladium implantation under alkaline conditions resulted in lowering the detected transmission percent. Therefore, Pd-C4 sample (UVA T% 0.9 & UVB T% 1) was exhibited by the highest UVA & UVB blocking percent of 99.1% and 99.0%, respectively, compared to the other three samples pristine cotton.
Figure S4 (supplementary file) demonstrated that, the decrement of transmission was significantly higher for the cationized cotton samples compared to that prepared from native cotton. Duplication of cationization percent and alkalinity were notably affected on diminishing of transmission percent, whereas, increment of concentration for palladium salt under alkaline conditions was shown to insignificantly affect the transmission. The estimated results showed that UVA T% & UVB T% were sharply decreased from 34.6 to 41.4% for cationized (50) cotton (UVA blocking 53.4%& UVB blocking 41.4%) to 2.7% & 1.8% for Pd-CC (50)2 (UVA blocking 97.3% & UVB blocking 98.2%) and superiorly to 0.7% and 0.5% for Pd-CC (50)3 (UVA & UVB blocking 99.3%) and Pd-CC (50)4 (UVA & UVB blocking 99.5%), respectively. Whereas, the lowest transmission percent of 0.4% for all the prepared samples was exhibited by Pd-CC (100)4 (the highest UVA & UVB blocking percent of 99.6%). These could affirm the effects of cationization, alkalinity and concentration of palladium precursor in implantation of greater amounts from palladium nanoclusters
UPF & UV protection rating were also evaluated in accordance to the estimated values of transmission percent (Table 4). The UPFs of native cotton, cationized (50) cotton and cationized (100) cotton fabrics were 1.3, 5.6 and 8.1, respectively. They were estimated to increase up to 103.4, 198.9 and 256.6 for Pd-C4, Pd-CC (50)4, and Pd-CC (100)4, respectively. The highest UPF was found in Pd-CC (100)4 to affirm the superior cationization effect on the efficient implantation of higher amounts of palladium nanoclusters within the fabric matrix to reveal higher blocking action for ultraviolet radiation, while, palladium nanoclusters were superiorly acted as excellent UV absorbers for preparation of UV protective textiles. Moreover, the demonstrated data for ultraviolet protection rating it could be declared that, all the samples that were prepared under alkaline conditions showed very good to excellent rating, for substantiation the excellency of palladium nanoclusters as UV blocking structures.
Compared with other reported approaches in literature the UV protection properties of cotton fabrics that were currently prepared via the self-implantation of palladium nanoclusters are extensively higher than for fabrics prepared in literature via the immobilization of metallic based ultraviolet absorbers like silver, gold, zinc, copper, or titanium (Ahmed et al. 2017a, b; Emam & Bechtold 2015; Emam, et al. 2016a, b; El-Hady et al. 2021), or via the impregnation of MOFs within the polymeric matrix of fabric (Emam & Abdelhameed 2017; Emam, et al. 2020). Therefore, the currently represented results suggested that palladium nanoclusters-coated fabrics could be expressed as potential candidates for ultraviolet shielding in textile functionalization, packaging applications and optoelectronics.
Durability
Table 5 represents the effect of repetitive washing cycles on the colorimetric data for the fabrics after successive immobilization of palladium nanoclusters. From the tabulated data it could be depicted that, cationization acted in preparation of higher durable fabrics, as even after 10 washing cycles, yellowness index of the cationized cotton (Pd-CC (100)4, 43.09 ± 2.09) was extremely higher than that of non-cationized fabric (Pd-C4, 7.34 ± 1.23). Whereas, with duplication of the cationization percentage from 50% up to 100%, color strength (K/S) was significantly higher for fabrics prepared under alkaline condition with higher concentration of palladium precursor (Pd-C4, 2.37 ± 0.41; Pd-CC (50)4, 7.80 ± 0.95; Pd-CC (100)4, 10.12 ± 1.32). These could affirm the effect of cationization for stabilized immobilization of palladium nanoclusters within the fabric matrix. Table 6 displayed the effect of washing on the ultraviolet protection results for the fabrics modified with palladium nanoclusters. The obtained results approved the effect of cationization in successive implantation of palladium nanoclusters, stabilized within the fabric matrix with repetitive washings to prepare highly durable/functionalized fabrics. Whereas, the results showed that, even after 10 washing cycles, the ultraviolet protection factor rating was decreased from excellent (UPF, Pd-CC (100)4, 256.6) to very good (UPF, Pd-CC (100)4, 39.6) for the cationized fabrics, while, it was significantly lowered from excellent (UPF, Pd-C4, 103.4) to good (UPF, Pd-C4, 23.5).
Conclusion
In the current study, for the first time, self-implantation of palladium nanoclusters within cotton fabrics was proceeded in order to act as strong ultraviolet absorbers to acquire the treated fabrics excellent ultraviolet protection potency with full shielding effects. The self-implanted palladium nanoclusters were immobilized within the polymeric matrix of both native and cationized cotton fabrics. For all the prepared specimens, the effects of the concentration of palladium precursor, pH, and cationization percentage on the particle size of the implanted nanoclusters, in addition to, the color coordinates, yellowness index, whiteness index, color strength, tensile strength, elongation percentage were systematically studied. The effect of repetitive washing cycles on the colorimetric data and the results of ultraviolet protection were also represented. From all of the illustrated data, it could be summarized that, palladium nanoclusters acted superiorly as strong ultraviolet shielding sites within the fabric matrix. The yellowness degree and UV protection potency were shown to follow the trend of Pd-CC (100)4 > Pd-CC (50)4 > > Pd-C4 > > Cationized (100) Cotton > Cationized (50) Cotton > native cotton. Pd-CC (100)4 exhibited good mechanical properties and excellent ultraviolet protection potentiality, that could be attributed to the effect of cationization in the stronger implantation of palladium nanoclusters within the fabrics. It could be eventually reported that, the textile industry should address the unique presented challenge for production of excellent ultraviolet protective cotton fabrics with full shielding effect, via self-implantation of palladium nanoclusters.
References
Abdelhameed RM, Kamel OM, Amr A, Rocha JO, Silva AM (2017) Antimosquito activity of a titanium–organic framework supported on fabrics. ACS Appl Mater Interfaces 9(27):22112–22120
Abdelhameed RM, El-Shahat M, Emam HE (2020) Employable metal (Ag & Pd)@ MIL-125-NH2@ cellulose acetate film for visible-light driven photocatalysis for reduction of nitro-aromatics. Carbohydr Polym 247:116695
Abdel-Mohdy F, Fouda MM, Rehan M, Aly A (2008) Repellency of controlled-release treated cotton fabrics based on cypermethrin and prallethrin. Carbohydr Polym 73(1):92–97
Adams BD, Chen A (2011) The role of palladium in a hydrogen economy. Mater Today 14(6):282–289
Ahmed HB, El-Hawary NS, Emam HE (2017a) Self-assembled AuNPs for ingrain pigmentation of silk fabrics with antibacterial potency. Int J Biol Macromol 105(Pt 1):720–729
Ahmed HB, El-Hawary NS, Emam HE (2017b) Self-assembled AuNPs for ingrain pigmentation of silk fabrics with antibacterial potency. Int J Biol Macromol 105:720–729
Ahmed HB, Emam HE, Mashaly HM, Rehan M (2018) Nanosilver leverage on reactive dyeing of cellulose fibers: color shading, color fastness and biocidal potentials. Carbohydr Polym 186:310–320
Ali HO, Al-Hindawi AM, Abdulkarim YI, Nugoolcharoenlap E, Tippo T, Alkurt FÖ, Karaaslan M (2021) Simulated and experimental studies of multi-band symmetric metamaterial absorber with polarization independent for radar applications. Chin Phys B. https://doi.org/10.1088/1674-1056/ac2b1c
Almeida, L. d. (2006). Functionalisation of textiles: future perspectives.
Ates ES, Unalan HE (2012) Zinc oxide nanowire enhanced multifunctional coatings for cotton fabrics. Thin Solid Films 520(14):4658–4661
Baban A, Yediler A, Ciliz NK (2010) Integrated water management and CP implementation for wool and textile blend processes. Clean-Soil, Air, Water 38(1):84–90
Baglioni P, Dei L, Fratoni L, Nosotro PL, Moroni M (2005) Process for the preparation of nano-and micro-particles of group II and transition metals oxides and hydroxides, the nano-and micro-particles thus obtained and their use in the ceramic, textile and paper industries. USA Patent, US20050175530A1
Cano OA, González CR, Paz JH, Madrid PA, Casillas PG, Hernández AM, Pérez CM (2017) Catalytic activity of palladium nanocubes/multiwalled carbon nanotubes structures for methyl orange dye removal. Catal Today 282:168–173
Charpentier P, Burgess K, Wang L, Chowdhury R, Lotus A, Moula G (2012) Nano-TiO2/polyurethane composites for antibacterial and self-cleaning coatings. Nanotechnology 23(42):425606
Durán N, Marcato PD, De Souza GI, Alves OL, Esposito E (2007) Antibacterial effect of silver nanoparticles produced by fungal process on textile fabrics and their effluent treatment. J Biomed Nanotechnol 3(2):203–208
El-Hady A, Farouk A, Saeed S, Zaghloul S (2021) Antibacterial and UV protection properties of modified cotton fabric using a Curcumin/TiO2 nanocomposite for medical textile applications. Polymers 13(22):4027
Emam HE, Abdelhameed RM (2017) Anti-UV radiation textiles designed by embracing with nano-MIL (Ti, In)–metal organic framework. ACS Appl Mater Interfaces 9(33):28034–28045
Emam HE, Ahmed HB (2019) Comparative study between homo-metallic & hetero-metallic nanostructures based agar in catalytic degradation of dyes. Inter J Biol Macromol 138:450–461
Emam HE, Bechtold T (2015) Cotton fabrics with UV blocking properties through metal salts deposition. Appl Surf Sci 357:1878–1889
Emam HE, Rehan M, Mashaly HM, Ahmed HB (2016a) Large scaled strategy for natural/synthetic fabrics functionalization via immediate assembly of AgNPs. Dye Pigment 133:173–183
Emam HE, Saleh N, Nagy KS, Zahran M (2016b) Instantly AgNPs deposition through facile solventless technique for poly-functional cotton fabrics. Inter J Biol Macromol 84:308–318
Emam HE, El-Hawary NS, Ahmed HB (2017) Green technology for durable finishing of viscose fibers via self-formation of AuNPs. Inter J Biol Macromol 96:697–705
Emam HE, Abdelhamid HN, Abdelhameed RM (2018) Self-cleaned photoluminescent viscose fabric incorporated lanthanide-organic framework (Ln-MOF). Dye Pigment 159:491–498
Emam HE, Darwesh OM, Abdelhameed RM (2020) Protective cotton textiles via amalgamation of cross-linked zeolitic imidazole framework. Indust Eng Chem Res 59(23):10931–10944
Emam HE, Ahmed HB, Gomaa E, Helal MH, Abdelhameed RM (2020a) Recyclable photocatalyst composites based on Ag 3 VO 4 and Ag 2 WO 4@ MOF@ cotton for effective discoloration of dye in visible light. Cellulose 27:7139–7715
Emam HE, Mikhail MM, El-Sherbiny S, Nagy KS, Ahmed HB (2020b) Metal-dependent nano-catalysis in reduction of aromatic pollutants. Environ Sci Poll Res 27(6):6459–6475
Emam HE, Saad NM, Abdallah AE, Ahmed HB (2020c) Acacia gum versus pectin in fabrication of catalytically active palladium nanoparticles for dye discoloration. Inter J Biol Macromol 156:829–840
Fei B, Deng Z, Xin JH, Zhang Y, Pang G (2006) Room temperature synthesis of rutile nanorods and their applications on cloth. Nanotechnology 17(8):1927
Fürstner A (2008) Active metals: Preparation, Characterization, Applications. Wiley-VCH, Weinheim (ISBN: 978-3-527-61516-2)
Gandhi S, Subramani RHH, Ramakrishnan T, Sivabalan A, Dhanalakshmi V, Nair MG, Anbarasan R (2010) Ultrasound assisted one pot synthesis of nano-sized CuO and its nanocomposite with poly (vinyl alcohol). J Mater Sci 45(6):1688–1694
Gao Y-N, Wang Y, Yue T-N, Weng Y-X, Wang M (2021) Multifunctional cotton non-woven fabrics coated with silver nanoparticles and polymers for antibacterial, superhydrophobic and high performance microwave shielding. J Colloid Interf Sci 582:112–123
Goncalves G, Marques PA, Pinto RJ, Trindade T, Neto CP (2009) Surface modification of cellulosic fibres for multi-purpose TiO2 based nanocomposites. Compos Sci Technol 69(7–8):1051–1056
Gorenšek M, Recelj P (2009) Reactive dyes and nano-silver on PA6 micro knitted goods. Text Res J 79(2):138–146
Gulrajani M, Gupta D, Periyasamy S, Muthu S (2008) Preparation and application of silver nanoparticles on silk for imparting antimicrobial properties. J Appl Polym Sci 108(1):614–623
Hashem M, Refaie R, Hebeish A (2005) Crosslinking of partially carboxymethylated cotton fabric via cationization. J Cleaner Production 13(9):947–954
Hashem M, Refaie R, Goli K, Smith B, Hauser P (2009) Enhancement of wrinkle free properties of carboxymethylated cotton fabric via ionic crosslinking with poly(vinylpyrrolidone). J Indus Text 39(1):57–80
Hassabo AG, El-Naggar ME, Mohamed AL, Hebeish AA (2019) Development of multifunctional modified cotton fabric with tri-component nanoparticles of silver, copper and zinc oxide. Carbohydr Polym 210:144–156
Hewson M (1994) Formaldehyde in textiles. J Soc Dyers Colourists 110(4):140–142
Hojjati B, Sui R, Charpentier PA (2007) Synthesis of TiO2/PAA nanocomposite by RAFT polymerization. Polymer 48(20):5850–5858
Hughes R, Schubert W, Buss R (1995) Solid-state hydrogen sensors using palladium-nickel alloys: effect of alloy composition on sensor response. J Electrochem Soc 142(1):249
Ibrahim HM, Zaghloul S, Hashem M, El-Shafei A (2021) A green approach to improve the antibacterial properties of cellulose based fabrics using Moringa oleifera extract in presence of silver nanoparticles. Cellulose 28(1):549–564
Karatepe A, Akgöl O, Abdulkarim Y, Dalgac Ş, Awl H, Muhammadsharif F, Luo H (2021) A monopole microwave-assisted electrochemical sensor for the detection of liquid chemicals. Digest J Nanomater Biostruct 16(3):765–776
Khan MZ, Ashraf M, Hussain T, Rehman A, Malik MM, Raza ZA, Zia Q (2015) In situ deposition of TiO 2 nanoparticles on polyester fabric and study of its functional properties. Fiber Polymer 16(5):1092–1097
Kiwi J, Graetzel M (1979) Projection, size factors, and reaction dynamics of colloidal redox catalysts mediating light induced hydrogen evolution from water. J Am Chem Soc 101(24):7214–7217
Ko CH, Park JG, Park JC, Song H, Han S-S, Kim J-N (2007) Surface status and size influences of nickel nanoparticles on sulfur compound adsorption. Appl Surf Science 253(13):5864–5867
Lee E, Lee JM, Koo JH, Lee W, Lee T (2010) Hysteresis behavior of electrical resistance in Pd thin films during the process of absorption and desorption of hydrogen gas. Int J Hydrog Energy 35(13):6984–6991
Li Q, Chen SL, Jiang WC (2007a) Durability of nano ZnO antibacterial cotton fabric to sweat. J Appl Polym Sci 103(1):412–416
Li Y, Wu D-X, Hu J-Y, Wang S-X (2007b) Novel infrared radiation properties of cotton fabric coated with nano Zn/ZnO particles. Colloids Surf: Physicochem Eng Aspects 300(1–2):140–144
Li X, Ye J, Lin Y, Fan L, Pang H, Gong W, Ning G (2011) Facile synthesis and flame retardant performance of NaAl (OH) 2CO3 whiskers. Powder Technol 206(3):358–361
Lim B, Kobayashi H, Camargo PH, Allard LF, Liu J, Xia Y (2010) New insights into the growth mechanism and surface structure of palladium nanocrystals. Nano Res 3(3):180–188
Liu P, Zhao Y, Qin R, Mo S, Chen G, Gu L, Zang D (2016a) Photochemical route for synthesizing atomically dispersed palladium catalysts. Science 352(6287):797–800
Liu X, Zhao X, Zhu J, Xu J (2016b) One-pot synthesis of magnetic palladium–NiFe2O4–graphene oxide composite: an efficient and recyclable catalyst for Heck reaction. Appl Organomet Chem 30(5):354–359
Mackus AJ, Weber MJ, Thissen NF, Garcia-Alonso D, Vervuurt RH, Assali S, Kessels WM (2015) Atomic layer deposition of Pd and Pt nanoparticles for catalysis: on the mechanisms of nanoparticle formation. Nanotechnology 27(3):034001
Maneerung T, Tokura S, Rujiravanit R (2008) Impregnation of silver nanoparticles into bacterial cellulose for antimicrobial wound dressing. Carbohydr Polym 72(1):43–51
Mehta MD (2008) Nanotechnology and the developing world: lab-on-chip technology for health and environmental applications. Bull Sci Technol Soc 28(5):400–407
Meyer N, Devillers M, Hermans S (2015) Boron nitride supported Pd catalysts for the hydrogenation of lactose. Catal Today 241:200–207
Moon CH, Myung NV, Haberer ED (2014) Chemiresistive hydrogen gas sensors from gold-palladium nanopeapods. Appl. Physics Letters 105(22):223102
Nazari A, Montazer M, Mirjalili M, Nazari S (2013) Polyester with durable UV protection properties through using nano TiO2 and polysiloxane softener optimized by RSM. J Textile Inst 104(5):511–520
Perelshtein I, Applerot G, Perkas N, Guibert G, Mikhailov S, Gedanken A (2008) Sonochemical coating of silver nanoparticles on textile fabrics (nylon, polyester and cotton) and their antibacterial activity. Nanotechnology 19(24):245705
Rao GT, Babu B, Stella RJ, Manjari VP, Reddy CV, Shim J, Ravikumar R (2015) Synthesis and characterization of VO2+ doped ZnO–CdS composite nanopowder. J Mol Struct 1081:254–259
Rehan M, Hartwig A, Ott M, Gätjen L, Wilken R (2013) Enhancement of photocatalytic self-cleaning activity and antimicrobial properties of poly (ethylene terephthalate) fabrics. Surf Coat Technol 219:50–58
Rikkinen E, Santasalo-Aarnio A, Airaksinen S, Borghei M, Viitanen V, Sainio J, Krause AOI (2011) Atomic layer deposition preparation of Pd nanoparticles on a porous carbon support for alcohol oxidation. J Phys Chem C 115(46):23067–23073
Saengkiettiyut K, Rattanawaleedirojn P, Sangsuk S (2008) A study on antimicrobial efficacy of nano silver containing textile. J Nat Sci Special Issue Nanotechnology 7(1):33–36
Simončič B, Klemenčič D (2016) Preparation and performance of silver as an antimicrobial agent for textiles: a review. Text Res J 86(2):210–223
Subash AA, Chandramouli KV, Ramachandran T, Rajendran R, Muthusamy M (2012) Preparation, characterization, and functional analysis of zinc oxide nanoparticle-coated cotton fabric for antibacterial efficacy. J Texile Institute 103(3):298–303
Tan Y-J, Li J, Gao Y, Li J, Guo S, Wang M (2018) A facile approach to fabricating silver-coated cotton fiber non-woven fabrics for ultrahigh electromagnetic interference shielding. Appl Surf Sci 458:236–244
Tümen KU, Kıvrak B, Alkurt FÖ, Akyol M, Karaaslan M, Ekicibil A (2021) Synthesis and characterization of nanoparticles reinforced epoxy based advanced radar absorbing composites. J Mater Sci: Mater Electron 32(23):28007–28018
Vigneshwaran N, Kumar S, Kathe A, Varadarajan P, Prasad V (2006) Functional finishing of cotton fabrics using zinc oxide–soluble starch nanocomposites. Nanotechnology 17(20):5087
Wang M, Tang X-H, Cai J-H, Wu H, Shen J-B, Guo S-Y (2021) Construction, mechanism and prospective of conductive polymer composites with multiple interfaces for electromagnetic interference shielding: a review. Carbon 177:377–402
Welch CM (1988) Tetracarboxylic acids as formaldehyde-free durable press finishing agents: part I: catalyst, additive, and durability studies. Text Res J 58(8):480–486
Wong Y, Yuen C, Leung M, Ku S, Lam H (2006) Selected applications of nanotechnology in textiles. AUTEX Res J 6(1):1–8
Wu Z-C, Zhang Y, Tao T-X, Zhang L, Fong H (2010) Silver nanoparticles on amidoxime fibers for photo-catalytic degradation of organic dyes in waste water. Appl Surf Sci 257(3):1092–1097
Yang CQ, Xu L, Li S, Jiang Y (1998) Nonformaldehyde durable press finishing of cotton fabrics by combining citric acid with polymers of maleic acid. Text Res J 68(6):457–464
Yang CQ, Chen D, Guan J, He Q (2010) Cross-linking cotton cellulose by the combination of maleic acid and sodium hypophosphite. 1. Fabric wrinkle resistance. Indust Eng Chem Res 49(18):8325–8332
Zaluski L, Zaluska A, Tessier P, Ström-Olsen J, Schulz R (1995) Catalytic effect of Pd on hydrogen absorption in mechanically alloyed Mg2Ni, LaNi5 and FeTi. J Alloy Compound 217(2):295–300
Zhang H, Wang G, Sun G, Xu F, Li H, Li S, Fu S (2018) Facile synthesis of SiO2@ TiO2 hybrid NPs with improved photocatalytic performance. Micro Nano Letters 13(5):666–668
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Emam, H.E., Zaghloul, S. & Ahmed, H.B. Full ultraviolet shielding potency of highly durable cotton via self- implantation of palladium nanoclusters. Cellulose 29, 4787–4804 (2022). https://doi.org/10.1007/s10570-022-04567-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-022-04567-w