Abstract
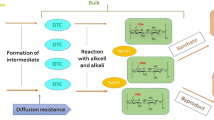
Cellulose dissolution in the viscose process has been facilitated through derivatization by carbon disulphide (CS2) at xanthation stage by converting alkali cellulose (AC) to cellulose xanthate (CX). CX formation has been always accompanied with sulphur based byproducts formation as dictated by the mechanism published in earlier study (Gondhalekar et al. (Cellulose 26 3 1595–1604, 2019)). The sulphur byproducts formed during viscose synthesis are sodium sulphide (Na2S), sodium trithiocarbonate (Na2CS3: TTC) and other minor sulphur compounds. These byproducts continue to form during ripening process as dictated by time and temperature coupled with concentration of free caustic and CS2 present in the system. These byproducts get converted into sodium sulphate (Na2SO4), hydrogen sulphide (H2S), CS2 and other sulphurous compounds during spinning. Overall, uncontrolled ripening without parametric optimization adversely impacts raw material (RM) consumption and creates sustainability challenges. Overall optimization based on viscose process fundamental insights presented in this study will effectively help in achieving operational excellence by reducing rate of undesired reactions to improve RM specific consumption and will compliment overall sustainability efforts in viscose industry.








Similar content being viewed by others
References
Abramova LS, Trusova SP, Rogovin ZA (1982) Ripening of low-substitution cellulose xanthate solutions. Fibre Chem 13:327–328
Awe OW, Zhao Y, Nzihou A, Minh DP, Lyczko N (2017) A review of biogas utilisation, purification and upgrading technologies. Waste Biomass Valoriz 8(2):267–283
Barthelemy HL, Williams L (1945) Determination of total sulfur and of gamma number of viscose. Ind Eng Chem Anal 17(10):624
Butkova NT, Tokareva TN, Pakshver AB et al (1978) Khim. Volokna 36–37
Dautzenberg H, Philipp B (1972) The reaction processes of soda cellulose with carbon disulphide. Fibre Chem 3(5):488–497
Duveen RF (1997) Technology and its development in the viscose industry. Lenzinger Berichte 76:33
Easterwood M, Mueller WA (1960) Factors affecting viscose ripening. J Appl Polym Sci 4(10):16–24
Finger GG, Pakshver AB (1990) Effect of sulfur-containing byproducts in viscose on its properties and the process of fibre spinning. Fibre Chem 22(3):168–176
Gbadebo AY, Carmichael G (1987) Kinetics of hydrolysis and oxidation of carbon disulfide by hydrogen peroxide in alkaline medium and application to carbonyl sulfide. Environ Sci Technol 21(2):170–177
Gondhalekar SC, Mohite LV, Pawar PJ, Datta SM, Naik-Nimbalkar VS (2019b) A study of viscose quality by reduction of knots in slurry and alkali cellulose. Cellul Chem Technol 53(3–4):219–226
Gondhalekar SC, Pawar PJ, Dhumal SS, Thakre SS (2019a) Mechanism of xanthation reaction in viscose process. Cellulose 26(3):1595–1604
Hovenkamp SG (1963) Sodium dithiocarbonate as a by-product in xanthating reactions. a contribution to the chemistry of viscose. J Polym Sci Part c: Polym Symp 1:341–355
John LH (1967) Man-made fibres. Interscience, New York
Klemm D, Philpp B, Heinze T, Hewinze U, Wagenknecht W (1998) Comprehensive cellulose chemistry Functionalization of cellulose. Wiley, USA
Koutu BB, Bhagwat VW (1999) Kinetic study of xanthation reaction with various pulps in viscose process. J Polym Mater 16(3):259–264
Kraft G, Schelosky N (2000) Irradiation of Dissolving Pulp by Electron Beams. Lenzinger Berichte 65–70.
Lanieri DB, Olmos GV, Alberini IC, Maximino MG (2014) Rapid estimation of gamma number of viscose by UV spectrophotometry. O Papel: Revista Mensal De Tecnologia Em Celulose e Papel 75(2):60–65
Lewin M, Pearce EM (1998) Handbook of fiber chemistry. CRC Press
Luvishis AP, Butkova NT, Pakshver AB, Finger GG, Berestyuk GI (1983) Mechanism of the reaction of sodium sulfite with sulfur-containing compounds in viscose. Fibre Chem 15(2):124–127
Maia DC, Niklevicz RR, Arioli R, Frare LM, Arroyo PA, Gimenes ML, Pereira NC (2017) Removal of H2S and CO2 from biogas in bench scale and the pilot scale using a regenerable Fe-EDTA solution. Renew Energy 109:188–194
Malyshevskaya KA, Mazur NA, Lasygina OV, Bibina NS, Bel’kevich IP (1976) Cellulose xanthate and by products in the viscose determined by spectrophotometry. Fibre Chem 8(2):233–234
Nurminen M, Hernberg S (1984) Cancer mortality among carbon disulfide-exposed workers. J Occup Environ Med 26(5):341
Onogi S, Hayashi Y (1959) A rheological interpretation of the hottenroth index method for viscose. Text Res J 29(11):873–879
Pakshver AB (1981) Scientific-research work of the VNIIVproekt on viscose chemistry during fifty years. Fibre Chem 13(2):104–112
Pavlov P, Valtcheva E, Makaztchieva V, Lozanov E (1991) Kinetics of xanthogenation after high-temperature mercerization. Acta Polym 42(9):462–465
Philipp B, Dautzenberg H, Schumann J (1974) Oxidation reactions of the sulphide compounds in the viscose process. Fibre Chem 5(6):678–684
Rahman M (1971) Spectrophotometric determination of xanthate and total sulfur in viscose. Anal Chem 43(12):1614–1618
Rassolov OP, Finger GG (1981) Effect of alkali cellulose composition and xanthation temperature on the maximum possible degree of esterification of cellulose xanthate. Fibre Chem 13(4):238–240
Schwaighofer A, Zuckerstätter G, Schlagnitweit J, Sixta H, Müller N (2011) Determination of the xanthate group distribution on viscose by liquid-state 1 H NMR spectroscopy. Anal Bioanal Chem 400(8):2449–2456
Sollinger S, Voges M (1997) Simultaneous determination of the main constituents of viscose spinning solutions by visible near infrared spectroscopy. J Near Infrared Spectrosc 5(3):135–148
Treiber E (1985) Formation of fibers from cellulose solutions. Cellulose Chemistry and its applications (Ed.): Nevell, TP and Zeronian, SH., 456–457. Minnesota: E. Horwood
Wilcosky TC, Checkoway H, Marshall EG, Tyroler HA (1984) Cancer mortality and solvent exposures in the rubber industry. Am Ind Hyg Assoc J 45(12):809–811
Wronski M (1956) Theory of kinetics of xanthation reaction. J Polym Sci A Polym Chem 19(91):210–212
Acknowledgments
Authors SCG, SSD, PP and ST acknowledges Grasim Industries Ltd. for financial support for this project. Authors also acknowledges Analytical Science and Technology Division of ABSTCPL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest for the submitted work.
Human and animal rights
This research does not involve any investigation related to human participation or animals conducted by the authors. In this regard, authors assure compliance of this work with ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gondhalekar, S.C., Pawar, P.J., Dhumal, S.S. et al. Fate of CS2 in viscose process: a chemistry perspective. Cellulose 29, 1451–1461 (2022). https://doi.org/10.1007/s10570-021-04398-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-021-04398-1