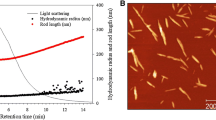
Abstract
Microcrystalline cellulose with high polydispersity was preferentially dissolved in 1-ethyl-3-methylimidazolium acetate (EMIMAC), an ionic liquid which has high ability for the dissolution and processing of cellulose. Either water, ethanol or acetonitrile as a non-solvent was carefully added to induce precipitation of cellulose fractions (Fig. 1). Ethanol or water addition led to quantitative precipitation of cellulose from EMIMAC solution. With the aprotic solvent acetonitrile, successive additions of this non-solvent promote fractional precipitation of cellulose into two coarse fractions. These two acetonitrile-induced precipitated cellulose fractions were revealed to have distinctly differing molecular weight profiles by size exclusion chromatography. The first precipitated fraction had proportionately higher molecular weight, more similar to the ethanol- and water-induced precipitates and the original cellulose, but with a relatively narrow polydispersity. The second fraction obtained by successive acetonitrile addition had relatively lower molecular weight and a similarly narrow polydispersity. Chemical characterisation confirmed this second fraction consisted of amorphous cellulose compared to other fractions containing crystalline celluloses. Thermogravimetric analysis also revealed the fractionated samples had relatively reduced thermal stability, consistent with the lower molecular weight of this fraction. Similarly, native cellulose can also be fractionated from chloride imidazolium-based ionic liquids with acetonitrile non-solvent to give low molecular weight fractions in 20–50% recovered yields. This relatively straightforward route to selective molecular weight fractionation of cellulose using acetonitrile non-solvent addition creates an opportunity for large-scale selective Mw fractionation of cellulose via ionic liquid processing.



Similar content being viewed by others
Notes
Cellulose was dissolved in EMIMAC solution at 80 °C under a nitrogen atmosphere to give a 2–8 w/v% solution. To this solution (30 g) maintained at 55 °C and non-solvent (100 mL), either water, ethanol or acetonitrile was added with stirring. The resulting suspensions were centrifuged to recover the precipitate. The filtrate was then combined with additional non-solvent with stirring and, in the case of further acetonitrile addition, centrifuging yielded a second precipitate. All precipitates were further washed with water prior to freeze drying and isolation.
References
Domínguez de María P (2014) Recent trends in (ligno)cellulose dissolution using neoteric solvents: switchable, distillable and bio-based ionic liquids. J Chem Technol Biotechnol 89(1):11–18
Fink HP et al (2001) Structure formation of regenerated cellulose materials from NMMO-solutions. Prog Polym Sci (Oxf) 26(9):1473–1524
Isik M, Sardon H, Mecerreyes D (2014) Ionic liquids and cellulose: dissolution, chemical modification and preparation of new cellulosic materials. Int J Mol Sci 15(7):11922–11940
Kathirgamanathan K (2009) Modifications of cellulose using ionic liquids. PhD thesis, School of Chemical Sciences. University of Auckland, Auckland
Kathirgamanathan K et al (2015) Two-dimensional FTIR as a tool to study the chemical interactions within cellulose-ionic liquid solutions. Int J Polym Sci 2015:1–9. doi:10.1155/2015/958653
Leskinen T et al (2013) Fractionation of lignocellulosic materials using ionic liquids: part 2. Effect of particle size on the mechanisms of fractionation. Ind Eng Chem Res 52(11):3958–3966
Meiland M, Liebert T, Heinze T (2011) Tailoring the degree of polymerization of low molecular weight cellulose. Macromol Mater Eng 296(9):802–809
Mischnick P, Momcilovic D (2010) Chemical structure analysis of starch and cellulose derivatives. Adv Carbohydr Chem Biochem 64:117–210. doi:10.1016/S0065-2318(10)64004-8
Schrems M et al (2011) Ionic liquids as media for biomass processing: opportunities and restrictions. Holzforschung 65(4):527–533
Stepan AM et al (2016) Cellulose fractionation with IONCELL-P. Carbohydr Polym 150:99–106
Wang H, Gurau G, Rogers RD (2012) Ionic liquid processing of cellulose. Chem Soc Rev 41(4):1519–1537
Acknowledgments
The authors wish to acknowledge the Biopolymer Network Ltd funded through the New Zealand Ministry of Business, Innovation and Employment for the provision of Ph.D. scholarship funding. The authors also wish to acknowledge the late Prof. Allan Easteal who was a Ph.D. supervisor, colleague and contributor to the outcomes of this research.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kathirgamanathan, K., Grigsby, W., Edmonds, N.R. et al. Molecular weight fractionation of high polydispersity native celluloses. Cellulose 24, 5261–5265 (2017). https://doi.org/10.1007/s10570-017-1422-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-017-1422-7