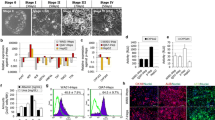
Abstract
The application of silver nanoparticles (AgNPs) in consumer products has been increasing rapidly over the past decades. Therefore, in vitro models capable of accurately predicting the toxicity of AgNPs are much needed. Hepatocyte-like cells (HLCs) derived from human induced pluripotent stem cells (iPSCs) represent an attractive alternative in vitro hepatotoxicity model. Yet, the use of iPSC-derived HLCs (iPSC-HLCs) for the study of nanoparticle toxicity has not been reported so far. In the present study, transcriptomic changes induced by varying concentrations (5–25 μg/ml) of AgNPs were characterized in iPSC-HLCs after 24-h exposure. AgNPs caused concentration-dependent gene expression changes in iPSC-HLCs. At all the concentrations, members of the metallothionein (MT) and the heat shock protein (HSP) families were the dominating upregulated genes, suggesting that exposure to AgNPs induced oxidative stresses in iPSC-HLCs and as a result elicited cellular protective responses in the cells. Functional analysis showed that the differentially expressed genes (DEGs) were majorly involved in the biological processes of metabolism, response to stress, and cell organization and biogenesis. Ingenuity Pathway Analysis revealed that cancer was at the top of diseases and disorders associated with the DEGs at all concentrations. These results were in accordance with those reported previously on hepatoma cell lines and primary hepatocytes. Considering the advantages iPSC-HLCs have over other liver cell models in terms of unlimited supply, consistency in quality, sustainability of function in long-term culture, and, more importantly, affordability of donor specificity, the results of the current study suggest that iPSC-HLCs may serve as a better in vitro model for liver nanotoxicology.





Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AgNPs:
-
Silver nanoparticles
- ANOVA:
-
Analysis of variance
- BP:
-
Biological process
- cDNA:
-
Complimentary DNA
- cRNA:
-
Complimentary RNA
- DAVID:
-
Database for Annotation, Visualization, and Integrated Discovery
- DEGs:
-
Differentially expressed genes
- DLS:
-
Dynamic light scattering
- FC:
-
Fold change
- FDR:
-
False discovery rate
- GO:
-
Gene ontology
- HCA:
-
Hierarchical cluster analysis
- HLCs:
-
Hepatocyte-like cells
- HSP:
-
Heat shock protein
- IPA:
-
Ingenuity Pathway Analysis
- iPSCs:
-
Induced pluripotent stem cells
- IVT:
-
In vitro transcription
- MT:
-
Metallothionein
- PCA:
-
Principal component analysis
- PHHs:
-
Primary human hepatocytes
- RMA:
-
Multi-array average
- ROS:
-
Reactive oxygen species
- SCENIHR:
-
Scientific Committee on Emerging and Newly Identified Health Risks
- TAC:
-
Transcriptome Analysis Console
- TEM:
-
Transmission electron microscopy
- UV-Vis:
-
Ultraviolet-visible
References
Ahamed M, Karns M, Goodson M, Rowe J, Hussain SM, Schlager JJ, et al. DNA damage response to different surface chemistry of silver nanoparticles in mammalian cells. Toxicol Appl Pharmacol. 2008;233(3):404–10. https://doi.org/10.1016/j.taap.2008.09.015.
Ahmadian E, Dizaj SM, Rahimpour E, Hasanzadeh A, Eftekhari A, Hosain Zadegan H, et al. Effect of silver nanoparticles in the induction of apoptosis on human hepatocellular carcinoma (HepG2) cell line. Mater Sci Eng C Mater Biol Appl. 2018;93:465–71. https://doi.org/10.1016/j.msec.2018.08.027.
Ale A, Rossi AS, Bacchetta C, Gervasio S, de la Torre FR, Cazenave J. Integrative assessment of silver nanoparticles toxicity in Prochilodus lineatus fish. Ecological Indicators. 2018;93:1190–8. https://doi.org/10.1016/j.ecolind.2018.06.023.
Brkic Ahmed L, Milic M, Pongrac IM, Marjanovic AM, Mlinaric H, Pavicic I, et al. Impact of surface functionalization on the uptake mechanism and toxicity effects of silver nanoparticles in HepG2 cells. Food Chem Toxicol. 2017;107(Pt A):349–61. https://doi.org/10.1016/j.fct.2017.07.016.
Connolly M, Fernandez-Cruz ML, Quesada-Garcia A, Alte L, Segner H, Navas JM. Comparative cytotoxicity study of silver nanoparticles (AgNPs) in a variety of rainbow trout cell lines (RTL-W1, RTH-149, RTG-2) and primary hepatocytes. Int J Environ Res Public Health. 2015;12(5):5386–405. https://doi.org/10.3390/ijerph120505386.
Davis SR, Cousins RJ. Metallothionein expression in animals: a physiological perspective on function. J Nutr. 2000;130(5):1085–8. https://doi.org/10.1093/jn/130.5.1085.
Dennis G Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, et al. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4(5):P3.
Donato MT, Tolosa L. Stem-cell derived hepatocyte-like cells for the assessment of drug-induced liver injury. Differentiation. 2019;106:15–22. https://doi.org/10.1016/j.diff.2019.02.004.
Dong L, Zhou X, Hu L, Yin Y, Liu J. Simultaneous size characterization and mass quantification of the in vivo core-biocorona structure and dissolved species of silver nanoparticles. J Environ Sci (China). 2018;63:227–35. https://doi.org/10.1016/j.jes.2017.10.010.
Faedmaleki F. F HS, Salarian AA, Ahmadi Ashtiani H, Rastegar H. Toxicity effect of silver nanoparticles on mice liver primary cell culture and HepG2 cell line. Iran J Pharm Res. 2014;13(1):235–42.
Faedmaleki F, Shirazi FH, Ejtemaeimehr S, Anjarani S, Salarian AA, Ahmadi Ashtiani H, et al. Study of silymarin and vitamin E protective effects on silver nanoparticle toxicity on mice liver primary cell culture. Acta Med Iran. 2016;54(2):85–95.
Fang H, Harris SC, Su Z, Chen M, Qian F, Shi L, et al. ArrayTrack: an FDA and public genomic tool. Methods Mol Biol. 2017;1613:333–53. https://doi.org/10.1007/978-1-4939-7027-8_13.
Farkas J, Christian P, Urrea JA, Roos N, Hassellov M, Tollefsen KE, et al. Effects of silver and gold nanoparticles on rainbow trout (Oncorhynchus mykiss) hepatocytes. Aquat Toxicol. 2010;96(1):44–52. https://doi.org/10.1016/j.aquatox.2009.09.016.
Foldbjerg R, Irving ES, Hayashi Y, Sutherland DS, Thorsen K, Autrup H, et al. Global gene expression profiling of human lung epithelial cells after exposure to nanosilver. Toxicol Sci. 2012;130(1):145–57. https://doi.org/10.1093/toxsci/kfs225.
Gao X, Liu Y. A transcriptomic study suggesting human iPSC-derived hepatocytes potentially offer a better in vitro model of hepatotoxicity than most hepatoma cell lines. Cell Biol Toxicol. 2017;33(4):407–21. https://doi.org/10.1007/s10565-017-9383-z.
Gao X, Topping VD, Keltner Z, Sprando RL, Yourick JJ. Toxicity of nano- and ionic silver to embryonic stem cells: a comparative toxicogenomic study. J Nanobiotechnology. 2017;15(1):31. https://doi.org/10.1186/s12951-017-0265-6.
Gerets HH, Tilmant K, Gerin B, Chanteux H, Depelchin BO, Dhalluin S, et al. Characterization of primary human hepatocytes, HepG2 cells, and HepaRG cells at the mRNA level and CYP activity in response to inducers and their predictivity for the detection of human hepatotoxins. Cell Biol Toxicol. 2012;28(2):69–87. https://doi.org/10.1007/s10565-011-9208-4.
Ghojavand S, Bagheri F, Mesrian Tanha H. Integrative meta-analysis of publically available microarray datasets of several epithelial cell lines identifies biological processes affected by silver nanoparticles exposure. Comp Biochem Physiol C Toxicol Pharmacol. 2019;216:67–74. https://doi.org/10.1016/j.cbpc.2018.11.003.
Green DR, Llambi F. Cell death signaling. Cold Spring Harb Perspect Biol. 2015;7(12). https://doi.org/10.1101/cshperspect.a006080.
Gupta SC, Sharma A, Mishra M, Mishra RK, Chowdhuri DK. Heat shock proteins in toxicology: how close and how far? Life Sci. 2010;86(11-12):377–84. https://doi.org/10.1016/j.lfs.2009.12.015.
Hewitt NJ, Lechon MJ, Houston JB, Hallifax D, Brown HS, Maurel P, et al. Primary hepatocytes: current understanding of the regulation of metabolic enzymes and transporter proteins, and pharmaceutical practice for the use of hepatocytes in metabolism, enzyme induction, transporter, clearance, and hepatotoxicity studies. Drug Metab Rev. 2007;39(1):159–234. https://doi.org/10.1080/03602530601093489.
Huang DW, Sherman BT, Tan Q, Kir J, Liu D, Bryant D, et al. DAVID bioinformatics resources: expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res. 2007;35(Web Server issue):W169–75. https://doi.org/10.1093/nar/gkm415.
Hunziker PE, Kagi JH. Isolation and characterization of six human hepatic isometallothioneins. Biochem J. 1985;231(2):375–82. https://doi.org/10.1042/bj2310375.
Kawata K, Osawa M, Okabe S. In vitro toxicity of silver nanoparticles at noncytotoxic doses to HepG2 human hepatoma cells. Environ Sci Technol. 2009;43(15):6046–51. https://doi.org/10.1021/es900754q.
Kermanizadeh A, Gaiser BK, Ward MB, Stone V. Primary human hepatocytes versus hepatic cell line: assessing their suitability for in vitro nanotoxicology. Nanotoxicology. 2013;7(7):1255–71. https://doi.org/10.3109/17435390.2012.734341.
Kim S, Ryu DY. Silver nanoparticle-induced oxidative stress, genotoxicity and apoptosis in cultured cells and animal tissues. J Appl Toxicol. 2013;33(2):78–89. https://doi.org/10.1002/jat.2792.
Kim YS, Kim JS, Cho HS, Rha DS, Kim JM, Park JD, et al. Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague-Dawley rats. Inhal Toxicol. 2008;20(6):575–83. https://doi.org/10.1080/08958370701874663.
Kim YS, Song MY, Park JD, Song KS, Ryu HR, Chung YH, et al. Subchronic oral toxicity of silver nanoparticles. Part Fibre Toxicol. 2010;7:20. https://doi.org/10.1186/1743-8977-7-20.
Lara HH, Garza-Trevino EN, Ixtepan-Turrent L, Singh DK. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J Nanobiotechnology. 2011;9:30. https://doi.org/10.1186/1477-3155-9-30.
Lu J, Einhorn S, Venkatarangan L, Miller M, Mann DA, Watkins PB, et al. Morphological and functional characterization and assessment of iPSC-derived hepatocytes for in vitro toxicity testing. Toxicol Sci. 2015;147(1):39–54. https://doi.org/10.1093/toxsci/kfv117.
Mann DA. Human induced pluripotent stem cell-derived hepatocytes for toxicology testing. Expert Opin Drug Metab Toxicol. 2015;11(1):1–5. https://doi.org/10.1517/17425255.2015.981523.
Martin JD, Frost PC, Hintelmann H, Newman K, Paterson MJ, Hayhurst L, et al. Accumulation of silver in yellow perch (Perca flavescens) and northern pike (Esox lucius) from a lake dosed with nanosilver. Environ Sci Technol. 2018;52(19):11114–22. https://doi.org/10.1021/acs.est.8b03146.
Massarsky A, Abraham R, Nguyen KC, Rippstein P, Tayabali AF, Trudeau VL, et al. Nanosilver cytotoxicity in rainbow trout (Oncorhynchus mykiss) erythrocytes and hepatocytes. Comp Biochem Physiol C Toxicol Pharmacol. 2014;159:10–21. https://doi.org/10.1016/j.cbpc.2013.09.008.
McShan D, Ray PC, Yu H. Molecular toxicity mechanism of nanosilver. J Food Drug Anal. 2014;22(1):116–27. https://doi.org/10.1016/j.jfda.2014.01.010.
Nowrouzi A, Meghrazi K, Golmohammadi T, Golestani A, Ahmadian S, Shafiezadeh M, et al. Cytotoxicity of subtoxic AgNP in human hepatoma cell line (HepG2) after long-term exposure. Iran Biomed J. 2010;14(1-2):23–32.
Pourali P, Nouri M, Ameri F, Heidari T, Kheirkhahan N, Arabzadeh S, et al. Histopathological study of the maternal exposure to the biologically produced silver nanoparticles on different organs of the offspring. Naunyn Schmiedebergs Arch Pharmacol. 2020;393:867–78. https://doi.org/10.1007/s00210-019-01796-y.
Prasad RY, McGee JK, Killius MG, Suarez DA, Blackman CF, DeMarini DM, et al. Investigating oxidative stress and inflammatory responses elicited by silver nanoparticles using high-throughput reporter genes in HepG2 cells: effect of size, surface coating, and intracellular uptake. Toxicol In Vitro. 2013;27(6):2013–21. https://doi.org/10.1016/j.tiv.2013.07.005.
Ruttkay-Nedecky B, Nejdl L, Gumulec J, Zitka O, Masarik M, Eckschlager T, et al. The role of metallothionein in oxidative stress. Int J Mol Sci. 2013;14(3):6044–66. https://doi.org/10.3390/ijms14036044.
Sahu SC, Zheng J, Yourick JJ, Sprando RL, Gao X. Toxicogenomic responses of human liver HepG2 cells to silver nanoparticles. J Appl Toxicol. 2015;35(10):1160–8. https://doi.org/10.1002/jat.3170.
Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR). Opinion on nanosilver: safety, health and environmental effects and role in antimicrobial resistance. 2014. http://ec.europa.eu/health/scientific_committees/emerging/docs/scenihr_o_039.pdf. Accessed 29 Oct 2019.
Si-Tayeb K, Noto FK, Nagaoka M, Li J, Battle MA, Duris C, et al. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology. 2010;51(1):297–305. https://doi.org/10.1002/hep.23354.
Slikker W Jr, Andersen ME, Bogdanffy MS, Bus JS, Cohen SD, Conolly RB, et al. Dose-dependent transitions in mechanisms of toxicity: case studies. Toxicol Appl Pharmacol. 2004;201(3):226–94. https://doi.org/10.1016/j.taap.2004.06.027.
Sullivan GJ, Hay DC, Park IH, Fletcher J, Hannoun Z, Payne CM, et al. Generation of functional human hepatic endoderm from human induced pluripotent stem cells. Hepatology. 2010;51(1):329–35. https://doi.org/10.1002/hep.23335.
Sun X, Wang Z, Zhai S, Cheng Y, Liu J, Liu B. In vitro cytotoxicity of silver nanoparticles in primary rat hepatic stellate cells. Mol Med Rep. 2013;8(5):1365–72. https://doi.org/10.3892/mmr.2013.1683.
Tang J, Xiong L, Wang S, Wang J, Liu L, Li J, et al. Distribution, translocation and accumulation of silver nanoparticles in rats. J Nanosci Nanotechnol. 2009;9(8):4924–32. https://doi.org/10.1166/jnn.2009.1269.
Tang J, Xiong L, Zhou G, Wang S, Wang J, Liu L, et al. Silver nanoparticles crossing through and distribution in the blood-brain barrier in vitro. J Nanosci Nanotechnol. 2010;10(10):6313–7. https://doi.org/10.1166/jnn.2010.2625.
The Woodrow Wilson International Center. Consumer products inventory. http://www.nanotechproject.org/cpi/. Accessed 29 Oct 2019.
Theocharis SE, Margeli AP, Koutselinis A. Metallothionein: a multifunctional protein from toxicity to cancer. Int J Biol Markers. 2003;18(3):162–9. https://doi.org/10.5301/jbm.2008.196.
Wen H, Dan M, Yang Y, Lyu J, Shao A, Cheng X, et al. Acute toxicity and genotoxicity of silver nanoparticle in rats. PLoS One. 2017;12(9):e0185554. https://doi.org/10.1371/journal.pone.0185554.
Wong HR. Heat shock proteins. Facts, thoughts, and dreams. A. De Maio. Shock 11:1-12, 1999. Shock. 1999;12(4):323–5. https://doi.org/10.1097/00024382-199910000-00012.
Xin L, Wang J, Fan G, Che B, Wu Y, Guo S, et al. Oxidative stress and mitochondrial injury-mediated cytotoxicity induced by silver nanoparticles in human A549 and HepG2 cells. Environ Toxicol. 2016;31(12):1691–9. https://doi.org/10.1002/tox.22171.
Xu L, Li X, Takemura T, Hanagata N, Wu G, Chou LL. Genotoxicity and molecular response of silver nanoparticle (NP)-based hydrogel. J Nanobiotechnology. 2012;10:16. https://doi.org/10.1186/1477-3155-10-16.
You C, Han C, Wang X, Zheng Y, Li Q, Hu X, et al. The progress of silver nanoparticles in the antibacterial mechanism, clinical application and cytotoxicity. Mol Biol Rep. 2012;39(9):9193–201. https://doi.org/10.1007/s11033-012-1792-8.
Zhou B, Ho SS, Greer SU, Spies N, Bell JM, Zhang X, et al. Haplotype-resolved and integrated genome analysis of the cancer cell line HepG2. Nucleic Acids Res. 2019;47(8):3846–61. https://doi.org/10.1093/nar/gkz169.
Acknowledgments
The authors thank Drs. Marianne Miliotis-Solomotis and Mary E. Torrence for critical review and approval of the manuscript as well as for their support of this work. The authors also thank Dr. Michael A. Adams in the Office of Food Additive Safety, Center for Food Safety and Applied Nutrition, U.S. Food and Drug Administration for critical review and approval of the manuscript.
Funding
This work was supported by internal funds of the U.S. Food and Drug Administration.
Author information
Authors and Affiliations
Contributions
XG conceived and designed the study, carried out the experimental work, analyzed the data, and wrote the manuscript. RL participated in all stages of the experiments. RLS and JJY contributed to the conception of the study, critically reviewed the manuscript, and gave approval of the final version to be published. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Disclaimer
The findings and conclusions presented in this article are those of the authors and do not necessarily represent views, opinions, or policies of the U.S. Food and Drug Administration.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gao, X., Li, R., Sprando, R.L. et al. Concentration-dependent toxicogenomic changes of silver nanoparticles in hepatocyte-like cells derived from human induced pluripotent stem cells. Cell Biol Toxicol 37, 245–259 (2021). https://doi.org/10.1007/s10565-020-09529-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-020-09529-1